Gemology Videos
Inclusions
Solid inclusions in Sapphire
Sapphire
Surface Features on Sapphire
Gemology Tools
Optical
Gemstone Treatments
Sapphire – Heat Treatment
General
Discussions
Advertisements

 Garnet is often thought of as red, but these gemstones come in almost any color and are popular for jewelry of all kinds. If you want to buy this January birthstone, that’s great news. In the gem world, the garnet family is one of the most interesting. It’s not a single species, but rather a group of different species and varieties that make it up.
Garnet is often thought of as red, but these gemstones come in almost any color and are popular for jewelry of all kinds. If you want to buy this January birthstone, that’s great news. In the gem world, the garnet family is one of the most interesting. It’s not a single species, but rather a group of different species and varieties that make it up.

| Name | Garnet |
| Varieties | Almandine Garnet, Almandine-Pyrope, Rhodolite Garnet, Andradite, Demantoid Garnet, Melanite, Topazolite, Andradite-Grossular, Mali Garnet, Color Change Garnet, Gadolinium Gallium Garnet, Grossular Garnet, Hessonite, Hibschite, Hydrogrossular Garnet, Transvaal Jade, Tsavorite Garnet, Malaia Garnet (Malaya Garnet), Proteus, Pyrope Garnet, Chrome Pyrope, Pyrope-Spessartine, Umbalite, Spessartite Garnet, Mandarin Garnet, Uvarovite Garnet, Yttrium Aluminium Garnet |
| Colors | A very wide range. See “Garnet Varieties” and “Identifying Characteristics” below and listings for specific garnet species and varieties. |
| Heat Sensitivity | Some |
| Formula | A3B2Si3O12. A = Fe, Ca, Mn, Mg. B = Al, Fe, Ti, Cr. See “Identifying Characteristics” below. |
| Etymology | From the Latin granatus for “grain.” Many garnet deposits are small grains of red crystals in or on their host rock. |
| Fracture | Conchoidal |
| Hardness | 6.5-7.5 |
| Cleavage | None |
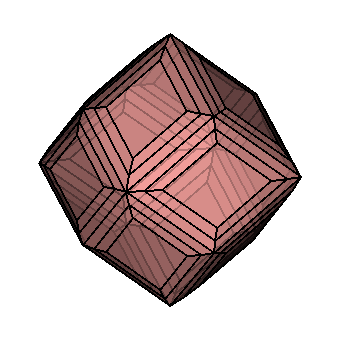
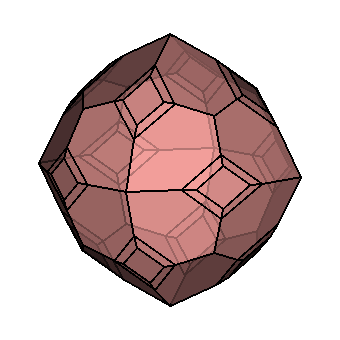
| Crystallography | Isometric. Trapezohedron and dodecahedron forms are common. Cube and octahedron forms are extremely rare. |
| Crystallographic Forms |
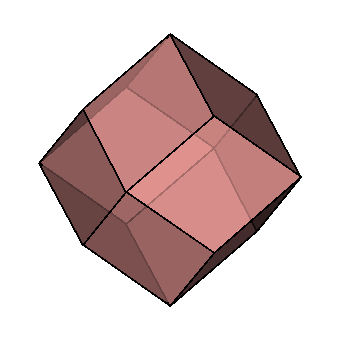 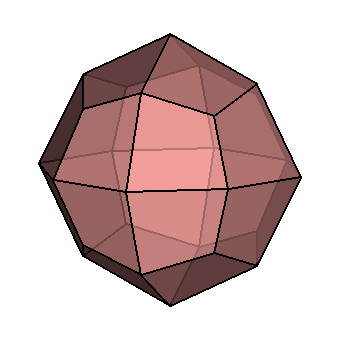 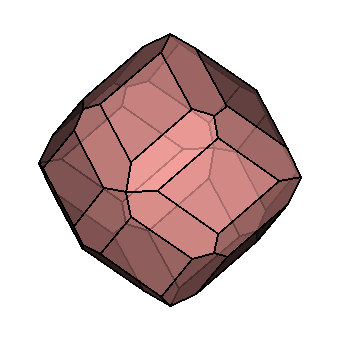   |
| Refractive Index | Varies by species, 1.72-1.95. See Gem Listings for specific varieties. |
| Birefringence | None |
| Dispersion | Varies by species, 0.014-0.057. See Gem Listings for specific varieties. |
| Luminescence | Most varieties are inert. Grossulars can show a variety of fluorescence. |
| Luminescence Present | Yes |
| Absorption Spectrum | See Gem Listings for specific varieties. |
| Pleochroism | None |
| Optics | Isotropic. Some garnets may show anomalous birefringence. See “Identifying Characteristics” below. |
| Luster | Vitreous, inclining to resinous in grossular, andradite, and some almandines. |
| Specific Gravity | 3.40-4.30 (See Gem Listings for specific varieties). |
| Transparency | Transparent to opaque |
| Birthstone | January |














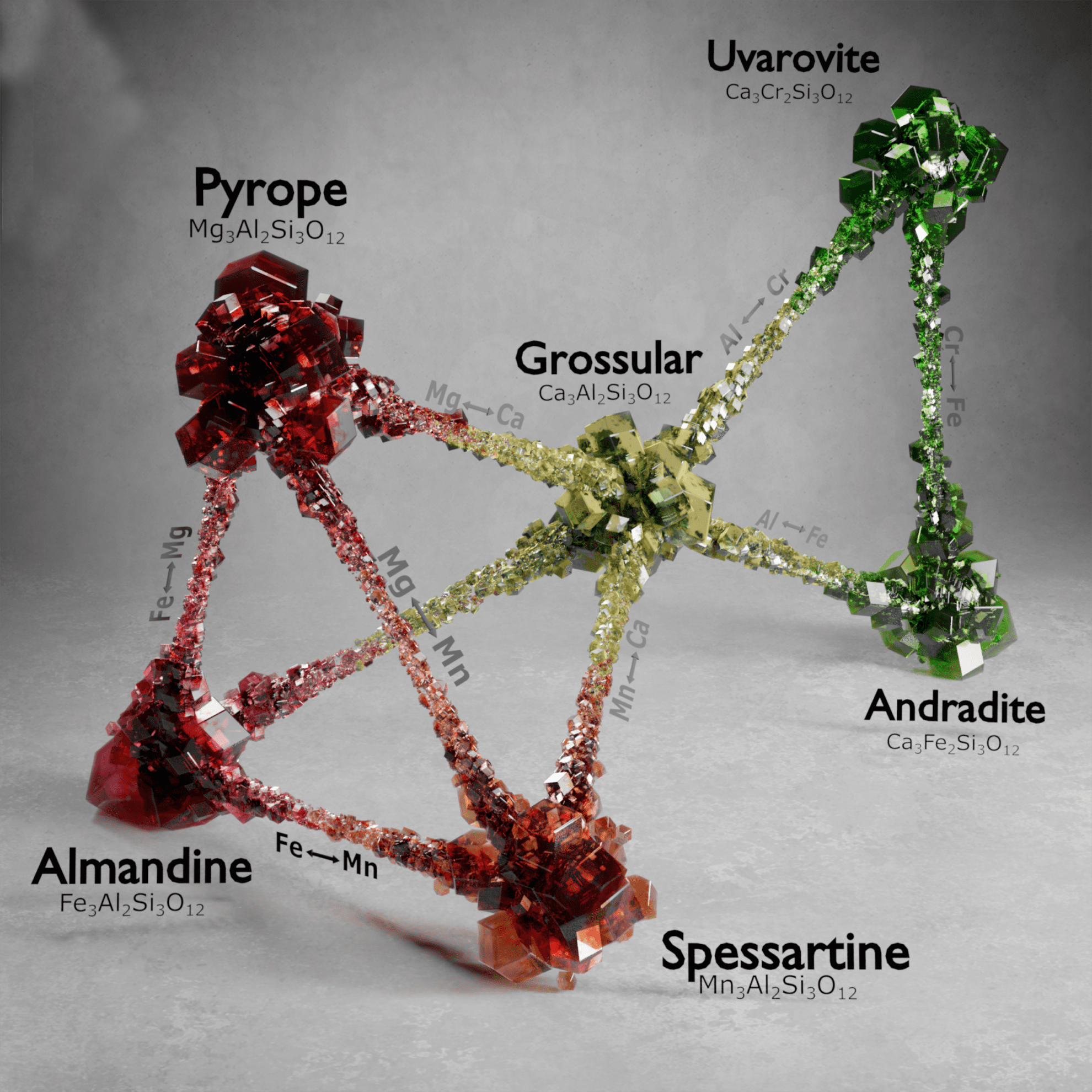
Garnet identification is difficult. Several new blends have been discovered in East Africa in the last fifty years. There’s no reason to suppose that all of the conceivable combinations have been found. We don’t know where gemologists will go in the future. Garnets have a lot in common at the molecular level, despite their differences. For those who aren’t interested in science, here’s a visualization that, while not scientifically sound, can assist illustrate the point. If your hand were a model of a garnet molecule, the arrangement of atoms represented by the palm would be shared by all garnets. The atoms represented by your fingers, on the other hand, are interchangeable. To put it another way, various atoms can live on your fingers while the palm stays the same. Any time the chemistry is altered, a new species emerges. Even if the structure and related features of a finger stay the same, changing the atoms produces a distinct species (or very nearly so).
As you can see, chemistry comes in a variety of forms. Despite this, they all have a similar core structure. In the isometric system, garnets crystallize. The trapezohedron and dodecahedron are the most popular shapes. Surprisingly, they are rarely found in the shapes of cubes or octahedrons, which are the most prevalent shapes of other isometric minerals. Garnets come in a variety of sizes and shapes, including enormous, granular, and tumbling pebbles. Rhodolite has been described as one part almandine and two parts pyrope by gemologists for decades. Garnets, on the other hand, are not so straightforward. Rhodolite stones, like all other garnets, contain some of the other species as well. Garnets are never as simple as having two elements, even if they are present in trace proportions. Furthermore, a solid-state series such as an almandine-pyrope blend does not imply that it is a mixture, Fe3Al2Si3O12 and Mg3Al2Si3O122. Instead, it denotes the presence of both Fe and Mg in the structure.
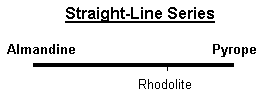 The almandine-pyrope and pyrope-spessartite series of garnets were once thought to constitute a straight-line sequence. However, this is insufficient to explain the increasingly complex mixtures we see. A two-dimensional graph with almandine, pyrope, and spessartite marking the three corners is a more helpful description.
The almandine-pyrope and pyrope-spessartite series of garnets were once thought to constitute a straight-line sequence. However, this is insufficient to explain the increasingly complex mixtures we see. A two-dimensional graph with almandine, pyrope, and spessartite marking the three corners is a more helpful description. 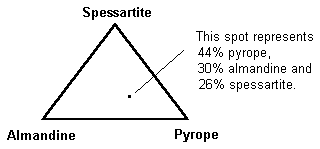 The chemistry of a gem is almost never found on one of the flat sides. It’s actually a point inside the triangle that indicates how much of each ingredient is present. A three-dimensional model is optimal for total accuracy. Andradite, grossular, and uvarovite can all be added to the formula in this way.
The chemistry of a gem is almost never found on one of the flat sides. It’s actually a point inside the triangle that indicates how much of each ingredient is present. A three-dimensional model is optimal for total accuracy. Andradite, grossular, and uvarovite can all be added to the formula in this way. 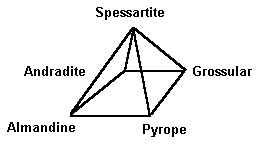 Grossular and andradite are found in practically all garnet blends, however they are less prevalent. This graph can be used to show how much of each of these species are present in a single specimen. Understanding garnet mixes is crucial for gemologists. The mixes of today’s garnets show a great deal of variation. Unless it’s a popular variant, garnets are usually named after their two principal species for identification purposes. Simply put, garnets are not simple two-species minerals. Gemologists used to classify garnets based on their chemical composition. This terminology is still in use. Pyralspites are garnets that have Al (aluminum) in the B position in their chemical formula (for pyrope, almandine, and spessartite). Ugrandites are garnets having Ca (calcium) in the A position (uvarovite, grossular, and andradite).
Grossular and andradite are found in practically all garnet blends, however they are less prevalent. This graph can be used to show how much of each of these species are present in a single specimen. Understanding garnet mixes is crucial for gemologists. The mixes of today’s garnets show a great deal of variation. Unless it’s a popular variant, garnets are usually named after their two principal species for identification purposes. Simply put, garnets are not simple two-species minerals. Gemologists used to classify garnets based on their chemical composition. This terminology is still in use. Pyralspites are garnets that have Al (aluminum) in the B position in their chemical formula (for pyrope, almandine, and spessartite). Ugrandites are garnets having Ca (calcium) in the A position (uvarovite, grossular, and andradite).

Garnet
Websites : www.giceylon.com www.bluesapphire.lk www.gemluck.com
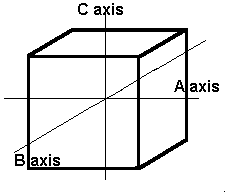
The isometric crystal system has three axes of the same length that intersect at 90º angles.
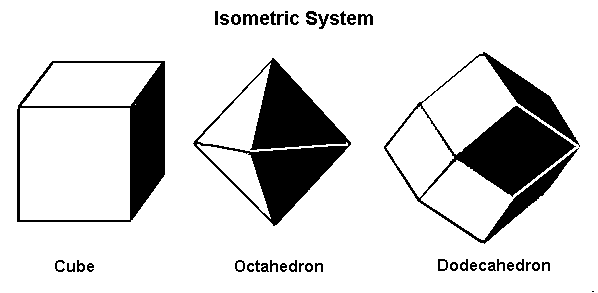
Minerals that form in the isometric system form in one of these three basic shapes.
 The Tetragonal System
The Tetragonal System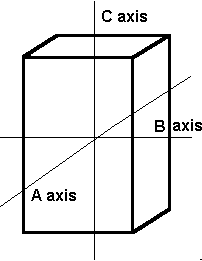
The tetragonal crystal system also has three axes. Axis C is longer than axes A and B, which are the same length.
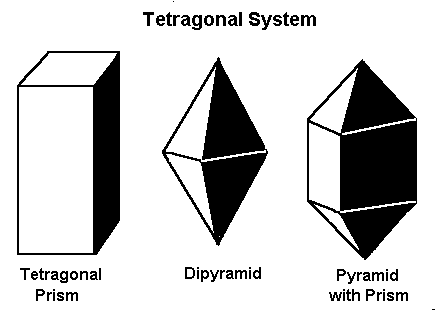
Minerals that form in the tetragonal system form in one of these three basic shapes.

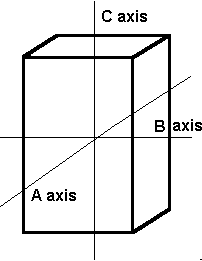
The orthorhombic system has three axes, each of which is a different length. These axes intersect at 90º angles.
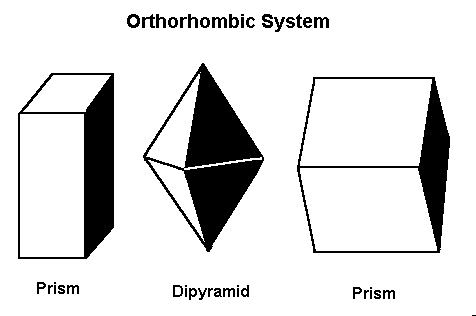
Minerals that form in the orthorhombic system form in one of these three basic shapes.
 The Monoclinic System
The Monoclinic System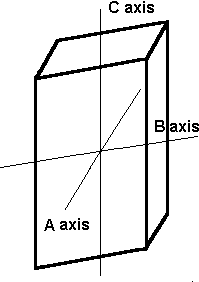
The axes in the monoclinic system are all of different lengths. The A and C axes intersect at 90º, but axis B does not.
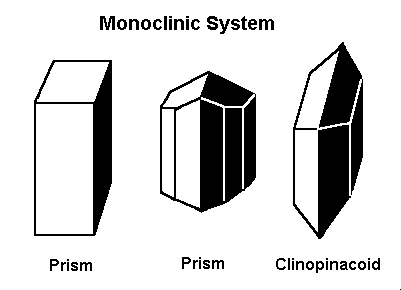
Gems that form in the monoclinic system form in one of these three basic shapes.
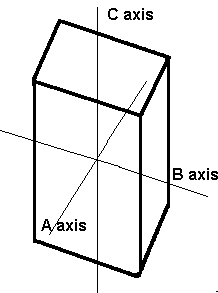
None of the axes in the triclinic system intersect at 90º and all are different lengths.
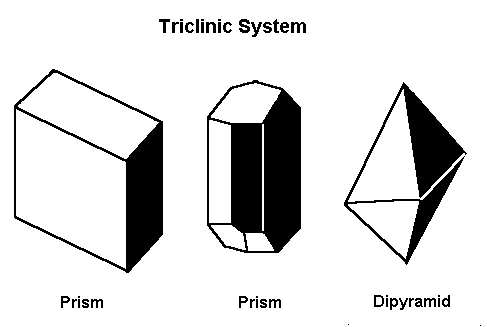
Gems that form in the triclinic system form in one of these three basic shapes.
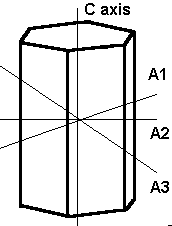
The hexagonal system has four axes. Three are equal in length and intersect at 60º. The longer C or vertical axis intersects the other shorter axes at 90º.
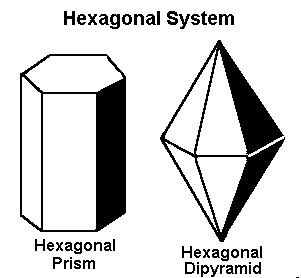
Gems that form in the hexagonal system form in one of these two basic shapes.
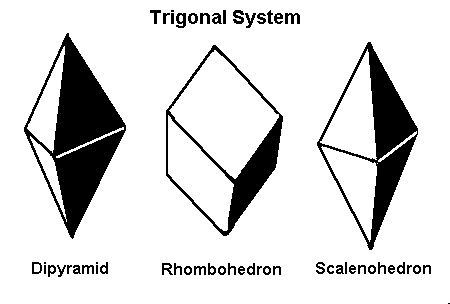
Most gem guides will list trigonal crystals as hexagonal. These crystals are sometimes distinguished from hexagonal crystals because of their appearance.




A gemstone’s optical properties can attract our eyes so it gives a cut and polished stone a unique character. However, the Physical Properties of Gemstones are also important. They determine a gem’s durability, guide gem cutters when they cut and set, and help us gemologists identify gemstones.
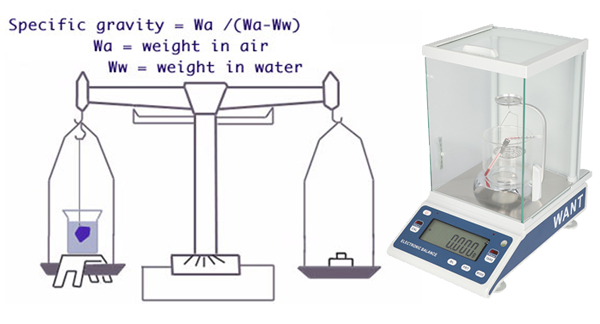 Specific gravity (SG) or density refers to how much something weighs in relation to its size. For example, a cubic centimeter of steel is much heavier than a cubic centimeter of styrofoam. SG is expressed as the relationship to an equivalent volume of water. For example, a gem with a specific gravity of 3 weighs three times as much as the same amount of water. In gemology, the terms specific gravity and density are interchangeable.
Specific gravity (SG) or density refers to how much something weighs in relation to its size. For example, a cubic centimeter of steel is much heavier than a cubic centimeter of styrofoam. SG is expressed as the relationship to an equivalent volume of water. For example, a gem with a specific gravity of 3 weighs three times as much as the same amount of water. In gemology, the terms specific gravity and density are interchangeable.
Specific Gravity Values of Popular Gemstones
Garnet 3.40-4.25
Corundum 3.97-4.03
Topaz 3.53-3.56
Diamond 3.51-3.53
Peridot 3.27-3.48
Tourmaline 2.84-3.10
Beryl 2.66-2.80
Quartz 2.63-2.68
Opal 1.99-2.25
Amber 1.05-1.096
As you see from the chart, few gems share a similar SG range. This makes SG one of every of the foremost useful gemstone physical properties you can have for identification. However, measuring specific gravity is difficult and time-consuming. Thus, you normally only conduct this test when necessary. (See “The Art and Science of Identifying Gemstones” for brand spanking new identification procedures that don’t typically require SG testing). Using specific gravity to Estimate Gem Weight When you’re buying and selling gems, knowing the SG of a gem can assist you to make the proper choice. For example, a one-carat opal with an SG of two would be much larger than a one-carat sapphire with an equivalent shape. Why? Because sapphire has an SG of 4. Let’s say you would like to seek out a replacement stone for a jewelry setting. While settings are measured in millimeters, gems are often sold by weight. If you order a one-carat sapphire to exchange a 1-carat opal, you’re certain an enormous surprise! If you recognize your gems well, you’ll estimate their weight by sight based on their SG.
Hardness and Toughness – Physical Properties of Gemstones The gemstone’s physical properties of hardness and toughness are often misunderstood. Their scientific meanings differ from their everyday meanings.
Hardness
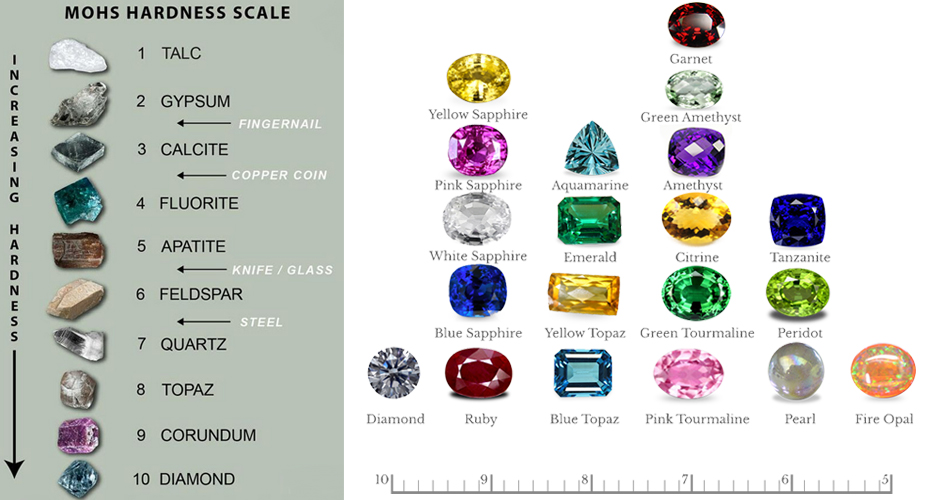
Scientifically, hardness is defined strictly because of the ability to resist scratching. Gemologists use the Moh’s Scale to define gemstone hardness. the scale runs from 1 to 10, with a selected mineral representing each number or unit. Diamond is the hardest at 10. Ruby comes in at 9, and so on. Each mineral on the scale will scratch softer stones, those below it. In turn, harder stones, those above them, will scratch it. Minerals of an equivalent hardness won’t scratch one another. 10 Diamond 9 Corundum 8 Topaz 7 Quartz 6 Feldspar 5 Apatite 4 Fluorite 3 Calcite 2 Gypsum 1 Talc
Moh’s Scale
Hardness has a very important effect on how well a gem will wear as jewelry. As a general rule (though exceptions abound), stones of hardness 7 or more will wear well. Since common dust consists largely of quartz (hardness 7), anything softer will eventually lose its polish. Even just wiping the dust off of a soft gem (below 7 in hardness) will cause fine scratches. Over time, these fine scratches accumulate and diminish the polish.
Toughness  Hardness has nothing to try and do with durability or toughness. Toughness or tenacity is defined because the ability to resist being hit. as an example, compare glass and wood. Scientifically, glass is far harder than wood. it’ll easily scratch the wood. However, you can hit a wooden board quite hard with a hammer without breaking it, yet glass will shatter with the slightest impact. So, while harder, glass isn’t nearly as tough as wood. You may not find toughness listed among gemstone physical properties in standard reference books. it’s no standard measurement procedures or units beyond verbal descriptions.
Hardness has nothing to try and do with durability or toughness. Toughness or tenacity is defined because the ability to resist being hit. as an example, compare glass and wood. Scientifically, glass is far harder than wood. it’ll easily scratch the wood. However, you can hit a wooden board quite hard with a hammer without breaking it, yet glass will shatter with the slightest impact. So, while harder, glass isn’t nearly as tough as wood. You may not find toughness listed among gemstone physical properties in standard reference books. it’s no standard measurement procedures or units beyond verbal descriptions.
Cleavage – Physical Properties of Gemstones 
Gemstone cleavage is a weak bond between the molecules of a mineral in certain planes in its crystal structure. you can best understand cleavage as a property of gemstones by comparing a gem to wood. Wood can split easily along the grain. However, splitting it across the grain is difficult. Some gems even have a direction along which they will easily split. this is often the cleavage plane. While cleavage affects toughness, it’s not related to hardness. Diamond is the hardest material known. However, since it’s cleavage planes, you’ll split it with a chunk of steel. Diamonds can break during normal wear. Cleavage fractures always run parallel to at least one plane of the first crystal’s surface. They’re always straight, flat, and difficult to examine if they’re inside the gem. you’ll spot them by the rainbow of colors that comes off a flat plane when light hits it at the right angle. Although hard to ascertain when small, cleavage fractures are vital for identifying and valuing a gem. Additionally, they indicate a weakness that would expand and ruin the gem.
Cleavage Descriptions
Gemologists describe cleavage in terms of how easily the material will part. the standard terms range from perfect, which separates very easily, to good, fair, and poor. you’ll come upon other terms, like imperfect. However, these are used less frequently. Another thing about descriptions is the number of cleavage planes. Some gems, like topaz, only have one direction of cleavage. Since topaz’s cleavage plane runs parallel to the bottom of the crystal, lapidaries usually cut it off at this axis. Thus, topaz is fairly durable despite its cleavage plane. Other stones, like feldspar, have cleavage on every crystal face. This makes cutting and setting problematic.
Please note that current cleavage terminology is a smaller amount than accurate. Take topaz, again. Because it’s described as having perfect cleavage, faceters realize it is often cut with minimum care. On the opposite hand, spodumene is an absolute monster to chop since it separates so easily. Still harder, diaspore comes during a class by itself. Yet, spodumene and diaspore are listed as having perfect cleavage, an equivalent to topaz and diamond. So, if you’re judging how well a stone will wear, look deeper into the cleavage than the easy listings.
Diamonds and topaz wear well. They’re commonly used as ring stones. However, kunzite, pink spodumene, and a few other gems would make very risky ring stones.
Fractures – Physical Properties of Gemstones 
Gemologists also describe how a mineral breaks in ways aside from along cleavage directions. They call this fracture. (Cleavage fractures are always flat, which makes them easy to spot and distinguish from other fractures). Although not always easy to call, fractures may offer you clues for gem identification. you’ll nearly always find fractures on the girdle or culet of a gem with a high-powered microscope. Of course, you’ll have better luck finding them on rough material. Fracture types include conchoidal, fibrous, splintery, granular, uneven, and hackly. The names are supported by what the fractures resemble. the foremost common sort of fracture is named “conchoidal”. You’ll see these on everything from glass to ruby.
Thus, they won’t help much with gem identification. However, discovering one among the less common fracture types would be a crucial clue. Keep in mind that fracture has nothing to try and do with the gemstone’s physical properties of hardness or toughness. Corundum is one among the foremost durable gems and it shares conchoidal fractures with glass, one among the foremost brittle. You can easily spot most of those fractures.
Conchoidal Typically, this fracture seems like an impact of a shell. If the fracture is a smaller amount complete, you’ll still see concentric banded lines during a curved section.
Fibrous Tiger’s eye gems have fibrous fractures.
Splintery Jade, ivory, and petrified wood (fossilized wood) often have splintery fractures. Be careful to not confuse fibrous and splintery. The difference is one among scale. Fibers are thin and fine, while splinters are usually thicker and coarser.
Granular fractures are common to lapis lazuli and maw-sit-sit.
Uneven Uneven fractures are often seen in sodalite and coral.
Hackly You’ll find hackly (sharp and jagged) fractures more commonly in metallic minerals.
Parting Like cleavage, parting occurs on a flat plane parallel to at least one of the crystal surfaces. Unlike cleavage, this results from gemstone twinning, where the crystal grew in overlapping layers. These layers have distinct thicknesses. Whereas a mineral with cleavage will have it in every specimen, parting only appears occasionally. It’s not present in every sample of a mineral species. for instance, you’ll find parts in some star rubies but not all of them. Star sapphires and rubies commonly contain parting. It’s somewhat common in chrysoberyl and quartz. You’ll usually see undercutting on the parting planes, as within the example pictured below. Undercutting represents a significant weakness during a gem and can greatly decrease its value and wearability.
 Stability – Physical Properties of Gemstones
Stability – Physical Properties of Gemstones
Stability refers to a gem’s ability to stay the same. Although most gems are very stable, don’t take that without any consideration. variety of things or sensitivities can affect the stability of certain gems. you ought to consider sensitivities generally, and light sensitivity especially, before you acquire a new gem, especially one you haven’t worked on. Leaving your beautiful, valuable, new acquisitions on your desk or during a bright case could cause them to change color or dry out.
Heat Sensitivity The most common gem stability problem is heat sensitivity. Gems don’t mind getting hot. Rather, the speed of change affects them. Thermal shock usually occurs during the cutting or setting of a gem. Lapidaries and metalsmiths need special training on the way to handle different gems to avoid thermal shock.
 Opals are so heat sensitive they even need special care when worn. you’ll cause them to crack by walking from a heated room into the winter cold or from an air-conditioned room into the complete summer sun. Since opals contain water, drying also can cause them to crack. To avoid cracking, store opals in an air-tight container or with a little piece of damp tissue. When wearing opals, keep them under your clothing if you’re going between environments with heat variations.
Opals are so heat sensitive they even need special care when worn. you’ll cause them to crack by walking from a heated room into the winter cold or from an air-conditioned room into the complete summer sun. Since opals contain water, drying also can cause them to crack. To avoid cracking, store opals in an air-tight container or with a little piece of damp tissue. When wearing opals, keep them under your clothing if you’re going between environments with heat variations.
Chemical Sensitivity  Certain chemicals can affect the look or composition of a gem with chemical sensitivity. This poses a serious problem for porous gems. for instance, turquoise and lapis lazuli can change color by absorbing oils from your skin. Therefore, don’t wear these gems while functioning on your car, painting, gardening, or doing anything where you can come in contact with chemicals. Certain acids can easily dissolve carbonates. These minerals include malachite, pearl, rhodochrosite, and marble. remember that a lot of common households and jewelry cleaners contain these acids.
Certain chemicals can affect the look or composition of a gem with chemical sensitivity. This poses a serious problem for porous gems. for instance, turquoise and lapis lazuli can change color by absorbing oils from your skin. Therefore, don’t wear these gems while functioning on your car, painting, gardening, or doing anything where you can come in contact with chemicals. Certain acids can easily dissolve carbonates. These minerals include malachite, pearl, rhodochrosite, and marble. remember that a lot of common households and jewelry cleaners contain these acids. 
Pearl is notoriously sensitive to chemicals. Always placed your pearl jewelry last when you’re getting dressed. Hairspray and atomized perfumes can ruin them.
Light Sensitivity 
The colors of some gems will fade if exposed to enough light. Such light-sensitive gems are called “evening stones,” since they’re best reserved for evening wear when they’re not exposed to sunlight. Even display case lights can damage light-sensitive gems. Thus, take care when displaying these gems for sale. (In addition, display lights can produce enough heat to dehydrate opals). Many gems suffer from light sensitivity to some degree. Some brown and gold topaz will fade slowly over time. As kunzite loses its color, it also loses a lot of value. Even within an equivalent species, sensitivity can vary. Blue spodumene, for instance, is somewhat light-sensitive. However, hiddenite (green spodumene) is so light-sensitive you ought to keep these rare specimens covered and only take them out for brief periods.
Artificially irradiated gems also can fade when exposed to light or heat.
Streak 
Optical properties largely determine a gem’s color. Streak, however, is one of the gemstone’s physical properties. It shows the color of a mineral without selective absorption live. To conduct a streak test, you powder a small little bit of a stone by rubbing the specimen across an unglazed porcelain tile or streak plate. Some stones have a streak color that differs considerably from what it shows under the light. for instance, most transparent colored stones, including emeralds, will show a colorless or white streak. Streak testing can help identify some stones. Hematite, an opaque metallic gemstone, features a reddish-brown streak, whereas hematite, a standard hematite imitation, features a brownish-black steak. However, don’t conduct this destructive test on finished stones. Test material in inconspicuous spots as a final resort only.
Magnetism Magnetism is one of the foremost misunderstood gemstone physical properties. The terms magnetism and magnetic refer to things possessing a magnetic field. Gemologists don’t use these terms to explain things that are attracted to magnets. Rather, they describe things that are magnets themselves. For instance, magnets attract ferrous metals. Hematite, a form of iron ore, would be attracted to a magnet. However, hematite itself isn’t magnetic, while hematine is. it’ll acquire metal objects.  Tests for identifying gemstone magnetism usually involve suspending the gem from a thread and holding it near a magnet. Although they work, you’ll put in a lot of labor for the small information you’ll receive. Keep this property in mind, but know there are other, much easier, and customary gem identification tests.
Tests for identifying gemstone magnetism usually involve suspending the gem from a thread and holding it near a magnet. Although they work, you’ll put in a lot of labor for the small information you’ll receive. Keep this property in mind, but know there are other, much easier, and customary gem identification tests.
Electrical Properties Minerals have a range of electrical properties.
Electroconductivity As the name implies, this is often the ability of a material to conduct electricity. Minerals with metallic bonding, like gold, silver, and copper, commonly possess this ability. a couple of minerals with partial metallic bonding are electrical semiconductors. Most gem minerals are nonconductors of electricity. However, there’s a crucial exception. Blue diamonds colored by artificial irradiation act as electrical insulators. Gemologists can distinguish them from naturally colored and synthetic blue diamonds by using thermal inertia meters.
Piezoelectricity  Some minerals, like colemanite, quartz, and tourmaline, generate electricity when placed under pressure. This property, piezoelectricity, is common in several minerals to varying degrees. When pressure is exerted at the ends, electricity will flow and make opposite positive and negative poles. due to this property, quartz and tourmaline have various industrial applications. for instance, thin slices of quartz can control frequencies for radios and watches. Some pressure gauges use tourmaline. Tourmaline was used to measure the blast pressure of the first atom bomb in 1945.
Some minerals, like colemanite, quartz, and tourmaline, generate electricity when placed under pressure. This property, piezoelectricity, is common in several minerals to varying degrees. When pressure is exerted at the ends, electricity will flow and make opposite positive and negative poles. due to this property, quartz and tourmaline have various industrial applications. for instance, thin slices of quartz can control frequencies for radios and watches. Some pressure gauges use tourmaline. Tourmaline was used to measure the blast pressure of the first atom bomb in 1945.
Pyroelectricity Some minerals generate an electrical current when heated. Examples include boracite, colemanite (also piezoelectric), rhodizite, and sphalerite.
Frictional Electricity  When rubbed, many gems commonly develop an electrostatic charge or frictional electricity. Notable examples include tourmaline and amber. If you rub them against something like wool, these gems gain enough charge to pick up ashes or small pieces of paper. the traditional Greeks recognized this quality in amber over 2,500 years ago. The word “electricity” comes from the Greek name for amber, Elektron.
When rubbed, many gems commonly develop an electrostatic charge or frictional electricity. Notable examples include tourmaline and amber. If you rub them against something like wool, these gems gain enough charge to pick up ashes or small pieces of paper. the traditional Greeks recognized this quality in amber over 2,500 years ago. The word “electricity” comes from the Greek name for amber, Elektron.
Thermal Conductivity  Some stones, notably crystalline gems, make excellent heat conductors. Because of their thermal conductivity, they’re going to draw heat far away from your fingers. Thus, they feel cool to the touch. Poor thermal conductors, like amber, glass, and plastic, feel warm to the touch. They don’t conduct heat far away from the body. If you concentrate on how a stone feels in your hand, you’ll often spot an imitation. When unsure, use a sensitive part of the body like your tongue or lips (but as long as you’ve recently cleaned the stone). Crystalline gemstone surfaces also will de-mist more rapidly than amorphous glass. Gemologists use thermal conductivity instruments to differentiate diamond, which conducts heat all right, from its simulants and imitations. Avoid drafts when testing, as they can change the readings. Thermal inertia meters measure how quickly the surface temperature of a gem changes when heated. This test also can help identify diamonds, since they need much higher thermal inertia than other gems.
Some stones, notably crystalline gems, make excellent heat conductors. Because of their thermal conductivity, they’re going to draw heat far away from your fingers. Thus, they feel cool to the touch. Poor thermal conductors, like amber, glass, and plastic, feel warm to the touch. They don’t conduct heat far away from the body. If you concentrate on how a stone feels in your hand, you’ll often spot an imitation. When unsure, use a sensitive part of the body like your tongue or lips (but as long as you’ve recently cleaned the stone). Crystalline gemstone surfaces also will de-mist more rapidly than amorphous glass. Gemologists use thermal conductivity instruments to differentiate diamond, which conducts heat all right, from its simulants and imitations. Avoid drafts when testing, as they can change the readings. Thermal inertia meters measure how quickly the surface temperature of a gem changes when heated. This test also can help identify diamonds, since they need much higher thermal inertia than other gems.
Hot Point Testing Thermal reaction testing, or hot point testing, also can distinguish real gems from imitations. You’ll use a pin heated over a flame to get smoke and odors from gem samples and show reactions to high heat, like melting or bubbling. Since melting is common to plastic, amber, wax, and resins, that result means your sample either is one among those materials or was surface treated with them. Odors can often be diagnostic. Tortoiseshell and a few corals emit a burning hair smell, while jet, fossilized coal, features a petroleum aroma. Amber produces a resinous smell and whitish smoke. Plastic imitations have an acrid, chemical odor. Odors also can reveal wax and plastic surface treatments applied to gems. For instance, turquoise should haven’t any smell. However, you’ll detect the odor of any treatment on the surface of the gem. Hot point testing is another destructive test. Test material in inconspicuous spots as the last resort only.

Physical Properties of Gemstones Article Composed by : සම්පත් සමරසේකර
Sampath Samarasekara
Chairman: Gemological Institute of Ceylon
Chairman: Youth Gem Professionals Association
Chairman: Sampath Gems
Chairman: Nanosoft Web Develop Company Direct WhatsApp: https://wa.me/message/2A4AUALYVQWRA1
Websites: www.giceylon.com www.bluesapphire.lk www.gemluck.com
Join with Gem and Jewellery Business Help and Discussion Group on Telegram: https://t.me/joinchat/Q5cLnxilNw4xAr8T3TumfA
All images and text content are Copyrighted ©️
Supreme TV Interview Link : https://www.facebook.com/754258918/videos/10158772094653919/

Our Practical Gemology Programme : https://www.youtube.com/watch?v=ipYrdsl1Kec&t=7s
Practical Gemology Course Content : https://www.giceylon.com/practical-gemology/
Some words About us and Practical Gem Business Gem trading is considered as one of the top 10 most lucrative businesses in the world, but surprisingly very easy to establish yourself providing you the right knowledge. In short it is a walk in the park with the right guidance. Not just classroom knowledge, the most important factor is practical knowledge. The way you identify the rough or polished stone end potential is the key.
Purchasing rough is the most cost effective and most rewarding method with profits from 10% to sometimes 500% this is why Many traders prefer to buy in rough. When doing so you need to be able to identify the following, Type of stone How many carats can be cut from the rough?
The color and clarity after cutting If it needs further treatment to enhance the beauty (Heat treatment) Potential market value and return This is where we come in, With over 40 years and 3 generations of experience in the world’s most profitable gem industry (Ceylon / Sri Lanka) we are able to guide you from how to work your way from the mines to the buyer.
අප ආයතනයේ ඉගෙනුම ලබන ශිෂ්ය ශිෂ්යාවන්ට ලබා දෙනු ලබන මෙම පොත ඔබට ලබා දෙන්නට සිතුවේ ඔබත් මැණික් හා ස්වර්ණාභරණ කර්මාන්තයට උනන්දුවක් දක්වන නිසාය.
ඔබටත් හැකි අයුරෙන් අන් අයට දැනුම බෙදා දෙන්න. එවිට එකිනෙකාට චෝදනා කරනවා වෙනුවට දියුණු ව්යාපාරිකයන් පිරිසක් හොඳ සමාජයක් බිහි වෙනවා ඇත.
අද සමාජ මාධ්ය තුළ දක්නට ලැබෙන්නේ අනෙකාට චෝදනා කරමින්, විශේෂයෙන් තමන්ගේ හැකියාව තේරුම් නොගෙන තමන්ට කළ නොහැකි දෙය අන් අය කරන විට ඔවුන්ට අපහාස කරමින් ගත කරන ජීවිතය.
මැණික් කර්මාන්තය යනු උතුම් රැකියාවකි. එහිදී එවැනි පසුගාමී සිතුවිලි සිත තුළ තබාගෙන මෙම රැකියාව සිදුකළ නොහැකිය. ඔබගේ වාසනාව උදාකරගත නොහැකිය. මැණික් ව්යාපාරය කිරීමට ඉතාම සැහැල්ලු මනසක්, ධනාත්මක මනසක් ගොඩනඟා ගන්න.
මා ව්යාපාරයට යොමු වන කාලයේ (2010) බාහිර සමාජයේ කිසිදු අයෙකු,දැනුම ලබාදීමට,හරි මාර්ගයක යැවීමට,විදේශ වෙළඳාම ගැන උපදෙස් හා උදව් කිරීමට සිටියේ නැත. බොහෝ අයගෙන් විමසුවත් මගහැරීම පිළිතුර විය.
මෙම අඩුපාඩු දැක මවිසින්ම ඒවාට විසඳුම් සොයා ව්යාපාරිකයින්ට දැනුම ලබාදිය යුතු යැයි මා යෝජනා කළ අවස්ථාවලදී පරිණත යැයි කියන බොහෝ ව්යාපාරිකයන් ඒවා සමච්චලයට ලක් කරන ලදී.
අද ලංකාව තුල මැණික් හා ස්වර්ණාභරණ ව්යාපාරයට ඇති භාධා සියල්ලම තේරුම්ගෙන අපි ජාත්යන්තරය දක්වා මැණික් හා ස්වර්ණාභරණ රට තුළ සිටම අලෙවි කරන්නෙමු.එහි දී රජයට හෝ ආයතන වලට චෝදනා කිරීමෙන් ඵලක් නොවන බව මතක තබා ගන්න. ඔබම ගැටළු වලට විසඳුම් සොයා ගත යුතුය.
මා නිතරම පවසන පරිදි ඔබටත් අන්තර්ජාලය හරහා ව්යාපාරය සාර්ථකව සිදු කළ හැක. මාහට වසර 12 ක් ගතවූ එම කර්තව්යය ඔබට ඉතාමත් කෙටි කාලයකින් සිදු කිරීමට අවශ්ය දැනුම හා අත්දැකීම් ලබාදීම මා නිතරම සිදු කරන්නෙමි.
නමුත් ඔබට ඉංග්රීසි භාෂාව හා පරිගණක දැනුම, නූතන තාක්ෂණය ගැන දැනුම නොමැතිව අන්තර්ජාලය හරහා ව්යාපාර සිදු කළ නොහැකිය.
මා කලින් පොත් ලබා දුන්නේ (31/05/2021) ඔබට අද ලෝකයේ අන්තර්ජාල ව්යපාර සිදුවන ආකාරය ගැන අවබෝධයක් ලබා දීමටය.
Aliexpress වැනි වෙබ් අඩවිවල තිබෙන දේවල් ebay හරහා විකිණීම සිදු කිරීමට අද හැකියාව ඇති ලෝකයක් බිහි වී ඇති නම් මැණික් කර්මාන්තයද එම ආකාරයෙන්ම දියුණු කළ හැක. මැණික් යනු එවැනි දැවැන්ත වෙබ් අඩවි වල එක product එකක් පමණි.
https://t.me/joinchat/Q5cLnxilNw4xAr8T3TumfA
ව්යාපාරිකයන් ලෙස අන් අයට කළ හැකි ලොකුම උදව්ව ලෙස මා දකින්නේ දැනුම හා අත්දැකීම බෙදා දීමයි.
මෙය ඉංග්රීසි භාෂාවෙන් ඇති එමෙන්ම අන්තර්ජාලයේ විකිණීමටඇති පොතක් නිසා අපගේ වෙබ් අඩවියෙන් දින කිහිපයක් ඇතුළත ඉවත් කරන්නෙමි.
ඔබත් බාගත ( Download ) කරගෙන අන් අයටත් බෙදා දෙන්න.PDF ලෙස ලෙස ඇති icon එබීමෙන් ඔබට එය ලබාගත හැක.
Click PDF icon to Download the Gemology Book
DOWNLOAD COMPANY PROFILE [learn_press_profile]