Gemology
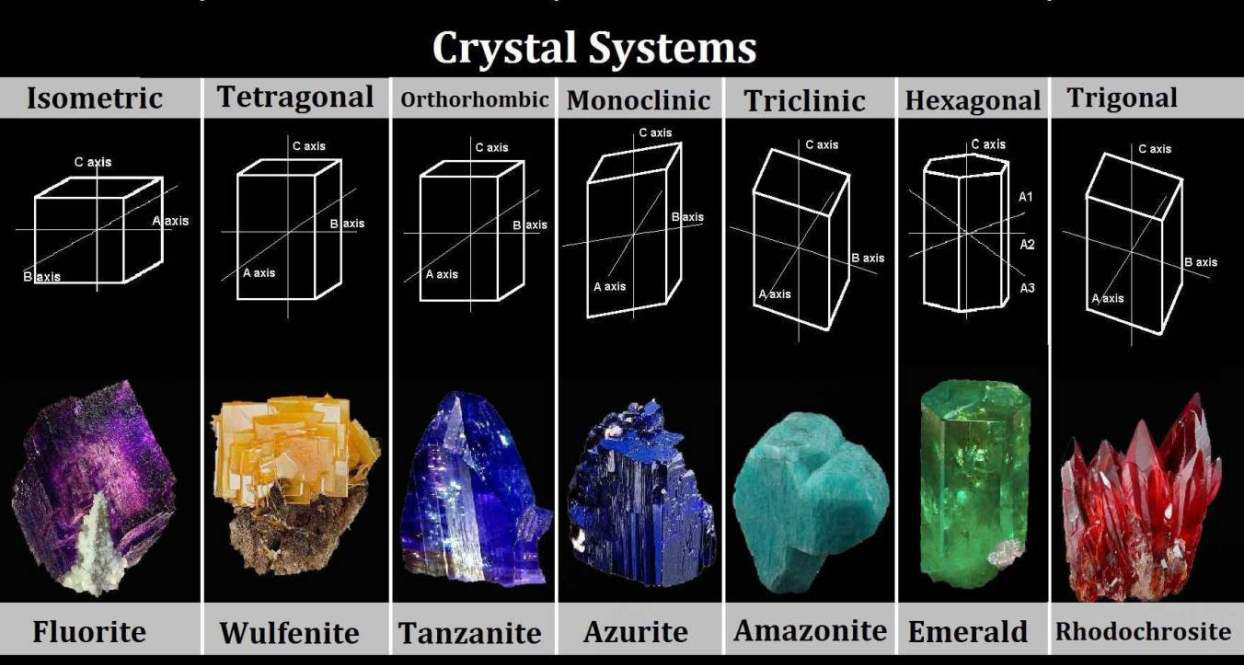
Crystal Systems
Crystal Systems
There are six crystal systems. All minerals form crystals in one of these six systems. Although you may have seen more than six shapes of crystals, they’re all variations of one of these six habits. Each system is defined by a combination of three factors:- How many axes it has.
- The lengths of the axes.
- The angles at which the axes meet.
The Isometric System
The first and simplest crystal system is the isometric or cubic system. It has three axes, all of which are the same length. The three axes in the isometric system all intersect at 90º to each other. Because of the equality of the axes, minerals in the cubic system are singly refractive or isotropic.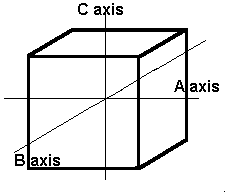
The isometric crystal system has three axes of the same length that intersect at 90º angles.
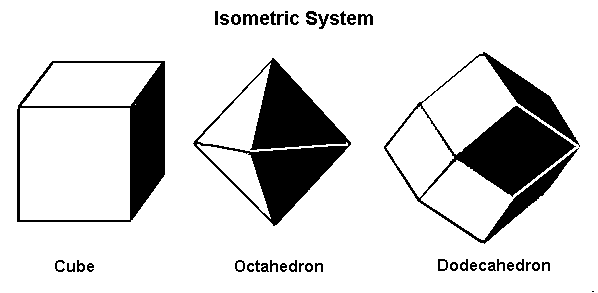
Minerals that form in the isometric system form in one of these three basic shapes.
 The Tetragonal System
The Tetragonal System
The tetragonal system also has three axes that all meet at 90º. It differs from the isometric system in that the C axis is longer than the A and B axes, which are the same length.
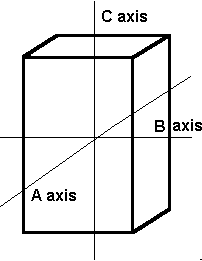
The tetragonal crystal system also has three axes. Axis C is longer than axes A and B, which are the same length.
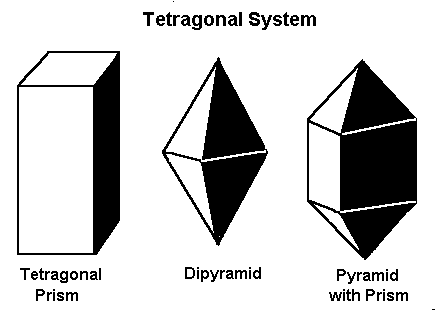
Minerals that form in the tetragonal system form in one of these three basic shapes.
The Orthorhombic System
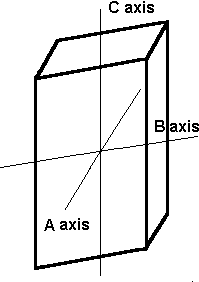
In this system, there are three axes, all of which meet at 90º to each other. However, all the axes are different lengths.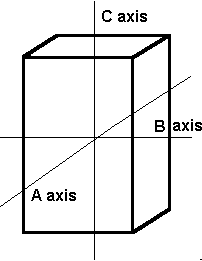
The orthorhombic system has three axes, each of which is a different length. These axes intersect at 90º angles.
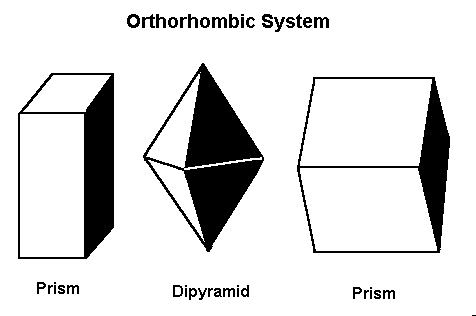
Minerals that form in the orthorhombic system form in one of these three basic shapes.
 The Monoclinic System
The Monoclinic System
The previously discussed crystal systems all have axes/sides that meet at 90º. In the monoclinic system, two of the axes, A and C, meet at 90º, but axis B does not. All axes in the monoclinic system are different lengths.
The axes in the monoclinic system are all of different lengths. The A and C axes intersect at 90º, but axis B does not.
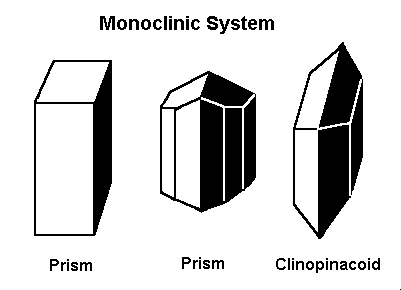
Gems that form in the monoclinic system form in one of these three basic shapes.
The Triclinic System
In the triclinic system, all the axes are different lengths. None of them meet at 90º.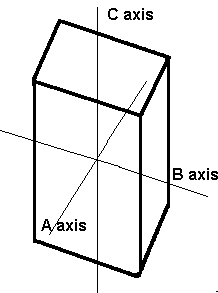
None of the axes in the triclinic system intersect at 90º and all are different lengths.
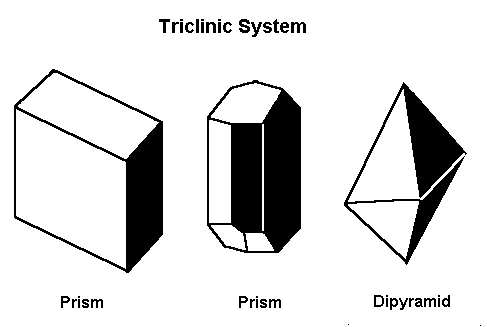
Gems that form in the triclinic system form in one of these three basic shapes.
The Hexagonal System
The crystal systems previously discussed represent every variation of four-sided figures with three axes. In the hexagonal system, we have an additional axis, which gives the crystals six sides. Three of these are equal in length and meet at 60º to each other. The C or vertical axis is at 90º to the shorter axes.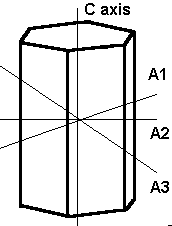
The hexagonal system has four axes. Three are equal in length and intersect at 60º. The longer C or vertical axis intersects the other shorter axes at 90º.
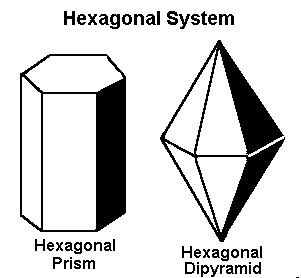
Gems that form in the hexagonal system form in one of these two basic shapes.
The Trigonal Subsystem
Mineralogists sometimes divide the hexagonal system into two crystal systems, the hexagonal and the trigonal, based on their external appearance. (Corundum, both ruby, and sapphire, is sometimes described as trigonal). However, for gemological purposes, the above six categories are sufficient.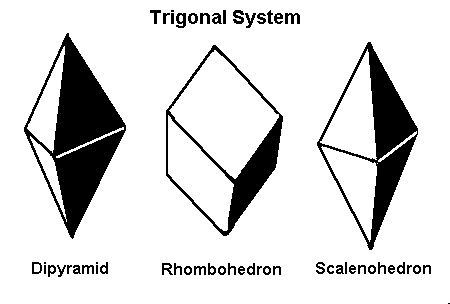
Most gem guides will list trigonal crystals as hexagonal. These crystals are sometimes distinguished from hexagonal crystals because of their appearance.
Gem Material Without Crystal Systems
Amorphous materials are not minerals. Thus, they don’t form in any of these crystal systems. Examples of amorphous materials used as gems include amber, glass (including obsidian), ivory, jet, moldavite, and opal. Some materials used as gems may contain crystals of minerals but can’t themselves be described as crystals because they don’t have a uniform crystal structure. These materials are called polycrystalline.