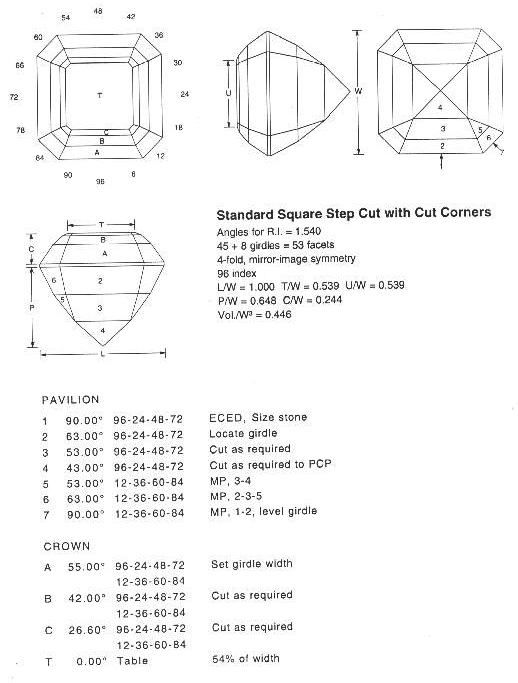
Facets in brilliant cuts are triangular and kite-shaped and radiate outward from the gem’s center. The brilliant-cut, as its name suggests, produces the highest scintillation of any cut.
Rectangular facets that ascend the crown and descend the pavilion in steps make up step cuts. Emerald and baguette cuts are examples of step cuts. These are popular because they highlight the color and clarity of the stone while also producing a slight sparkle.
Cem Cutting for Maximum Profit Article Composed by : සම්පත් සමරසේකර Sampath Samarasekara Chairman: Gemological Institute of Ceylon Chairman: Youth Gem Professionals Association Chairman: Sampath Gems Director: Ceylon Sapphire Gems and Jewels Director: Ceylon Gem Fair International Chairman: Nanosoft Web Develop Company Direct WhatsApp: https://wa.me/message/2A4AUALYVQWRA1
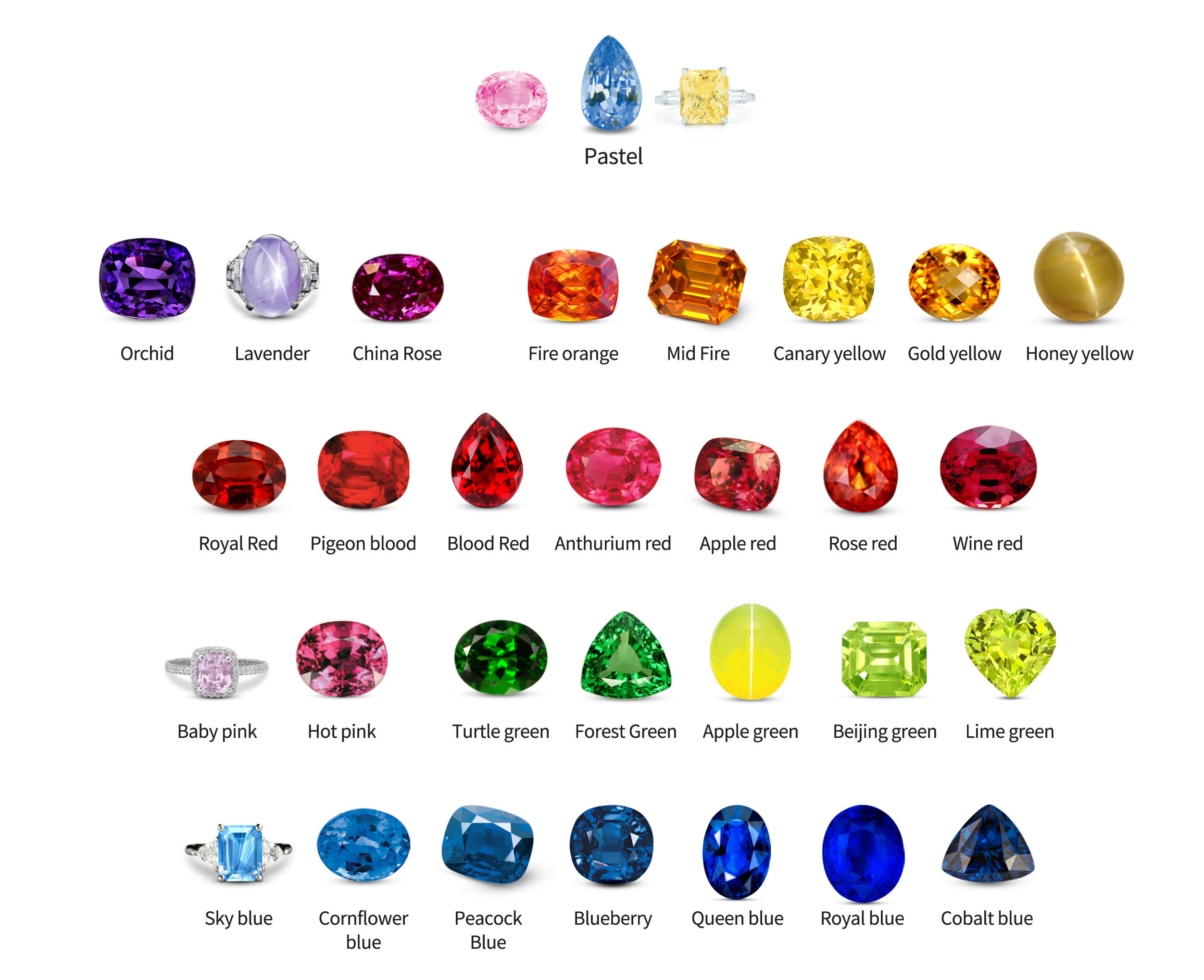
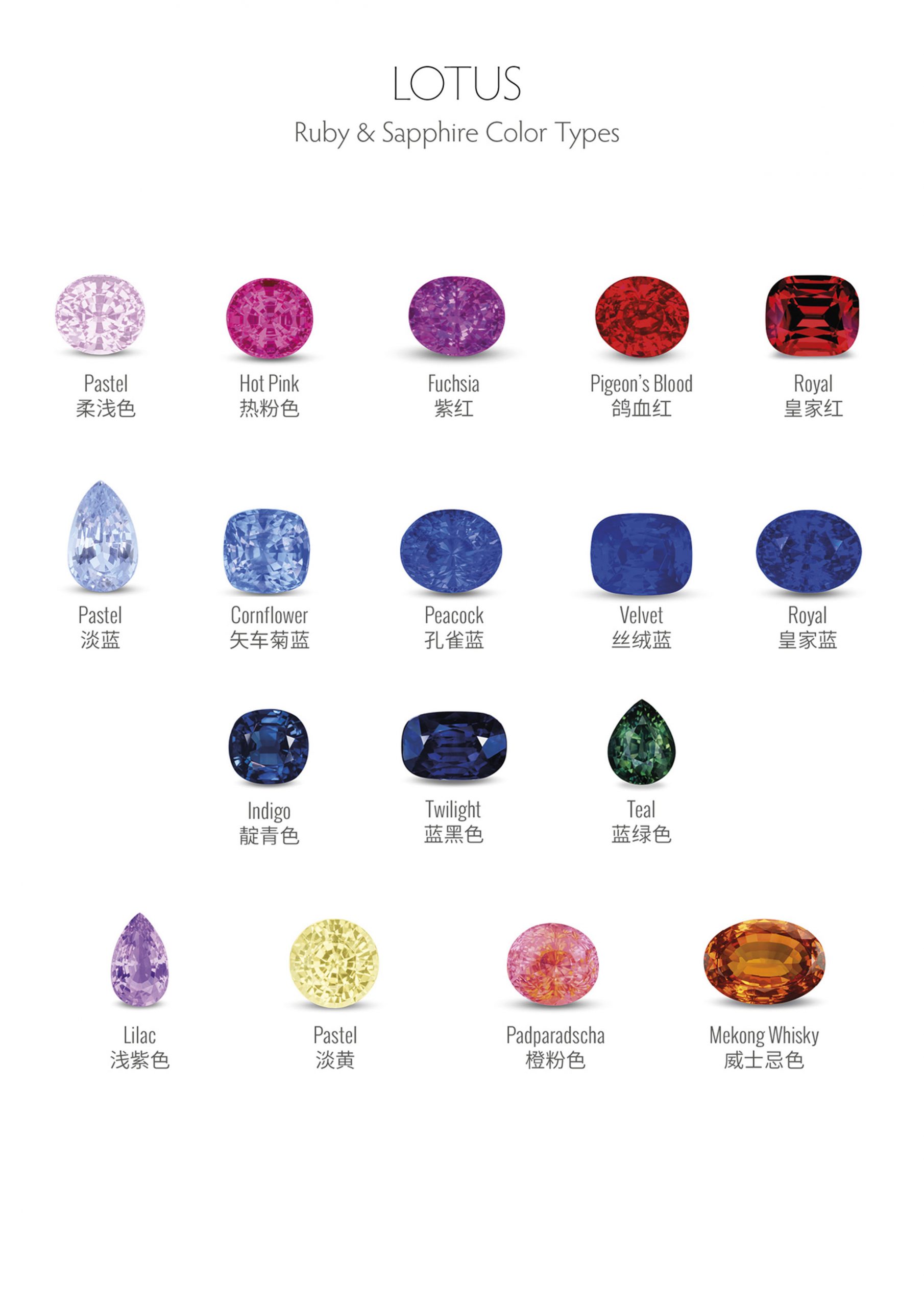
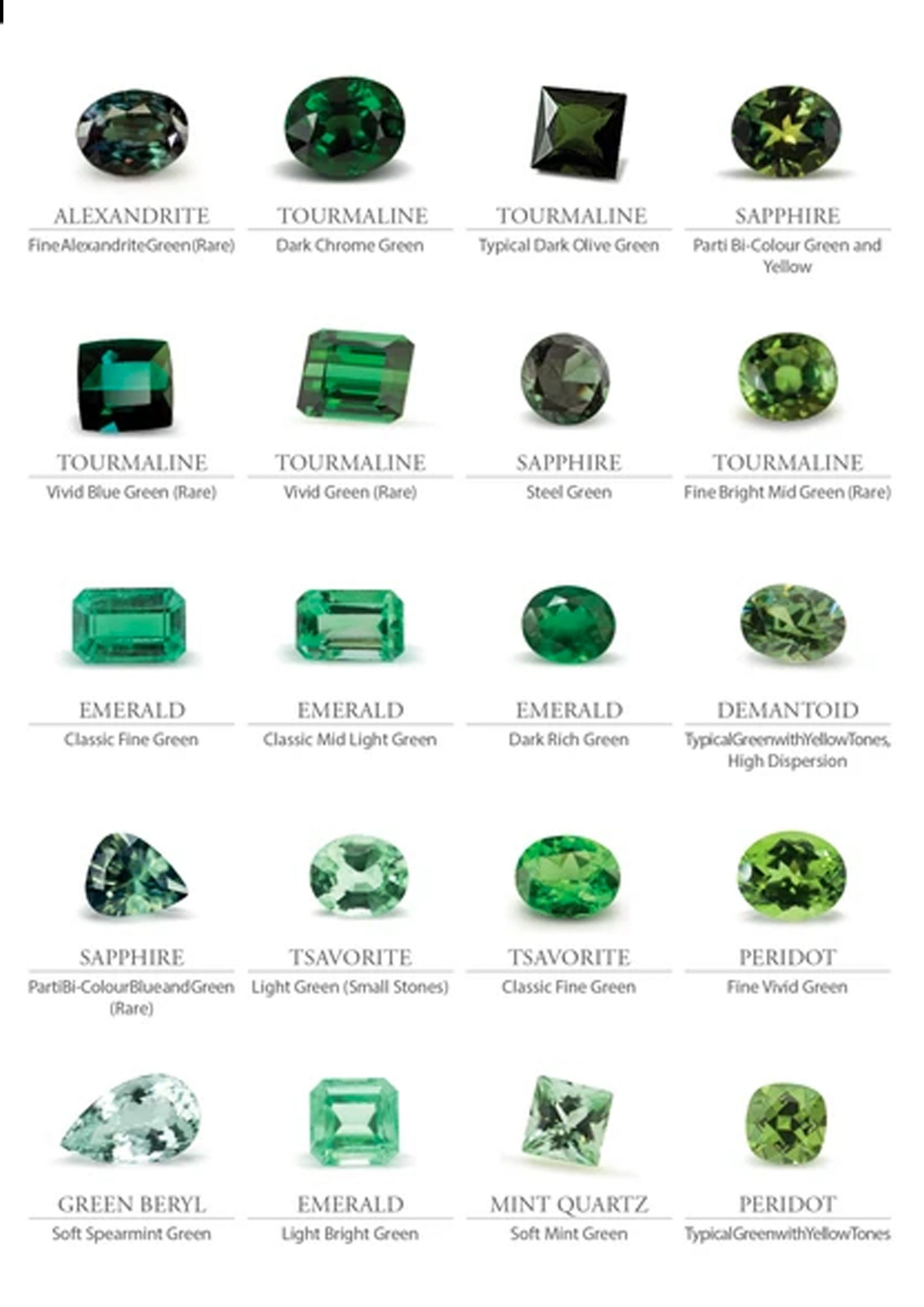
 Gemstone Color Grading – Hue, Tone, and Saturation
Gemstone Color Grading – Hue, Tone, and Saturation
Color is a critical factor in grading gemstones. Subtle variations in value can make a significant difference. Inexperienced gemologists must develop the ability to accurately describe colors. Numerous color description systems exist as a result of the highly refined methods developed by industries that deal with colors. Certain gemologists employ the Munsell system, which is widely used in the photographic industry.
Additionally, the gem industry has developed several systems. (It appears as though someone comes up with a new method every year.) Only the Gemological Institute of America (GIA) system, on the other hand, has achieved anything approaching widespread acceptance. Due to the fact that it is required of all GIA students, this system has the largest user base. Nonetheless, the colored stone industry has opposed its adoption as a standard. What is the issue? Establishing a standard will alter the current value of a large number of colored gems. For instance, the value of all rubies of a particular tone and hue may decline. This would be catastrophic for anyone in possession of such stones. The grading of colored stones is far too complicated for a straightforward system like that used for diamonds.
There are numerous ways to describe and grade the color of gemstones. We’ll begin with one of the most straightforward, but most effective, methods.
The Inverted “U” Method for Gemstone Color Description
The inverted “U” is one of the earliest methods of depicting the value of gems. While not as sophisticated as the GIA system, it effectively communicates how gem color affects value.
This method assigns a percentage to gem color ranging from 10% (colorless) to 90% (opaque).
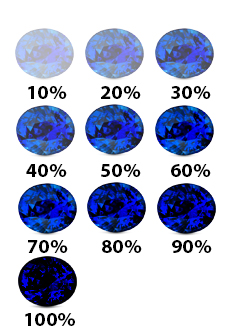
Colored stones achieve their greatest values between 40% and 60%, as shown in the graph below. Diamonds, on the other hand, have the opposite impact. The higher the value, the less color there is. The value of diamonds decreases as they become colored. The value of a fancy-colored diamond, on the other hand, skyrockets.
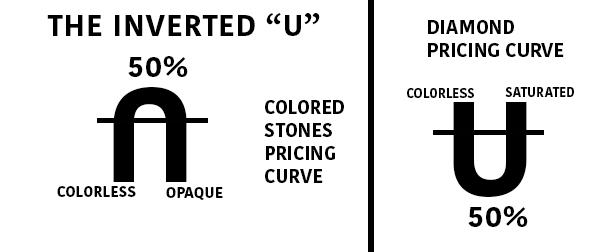
This gem color system, while handy, has certain limitations. To begin, it mixes tone and saturation, both of which have unique value impacts. Second, it ignores the color of the stone. Greenish blue, for example, may not be as valuable as pure blue. Third, these prices do not apply to all stones equally. For example, few aquamarines would ever reach 40% of their original value, whereas sapphires can reach 70% of their original value.
When grading a group of stones from a single source, on the other hand, the inverted “U” approach comes in handy. If you’ve received a large number of stones from a cutter, sort them by color grade using this approach. If you’re working with a group of iolite, for example, you can quickly check which ones have the highest value and which ones are outside the peak range.
The Gem Color System of the Gemological Institute of America (GIA)
One of the greatest systems for describing gem color is the GIA system. However, keep in mind that some of your business contacts might not be able to use or accept it.
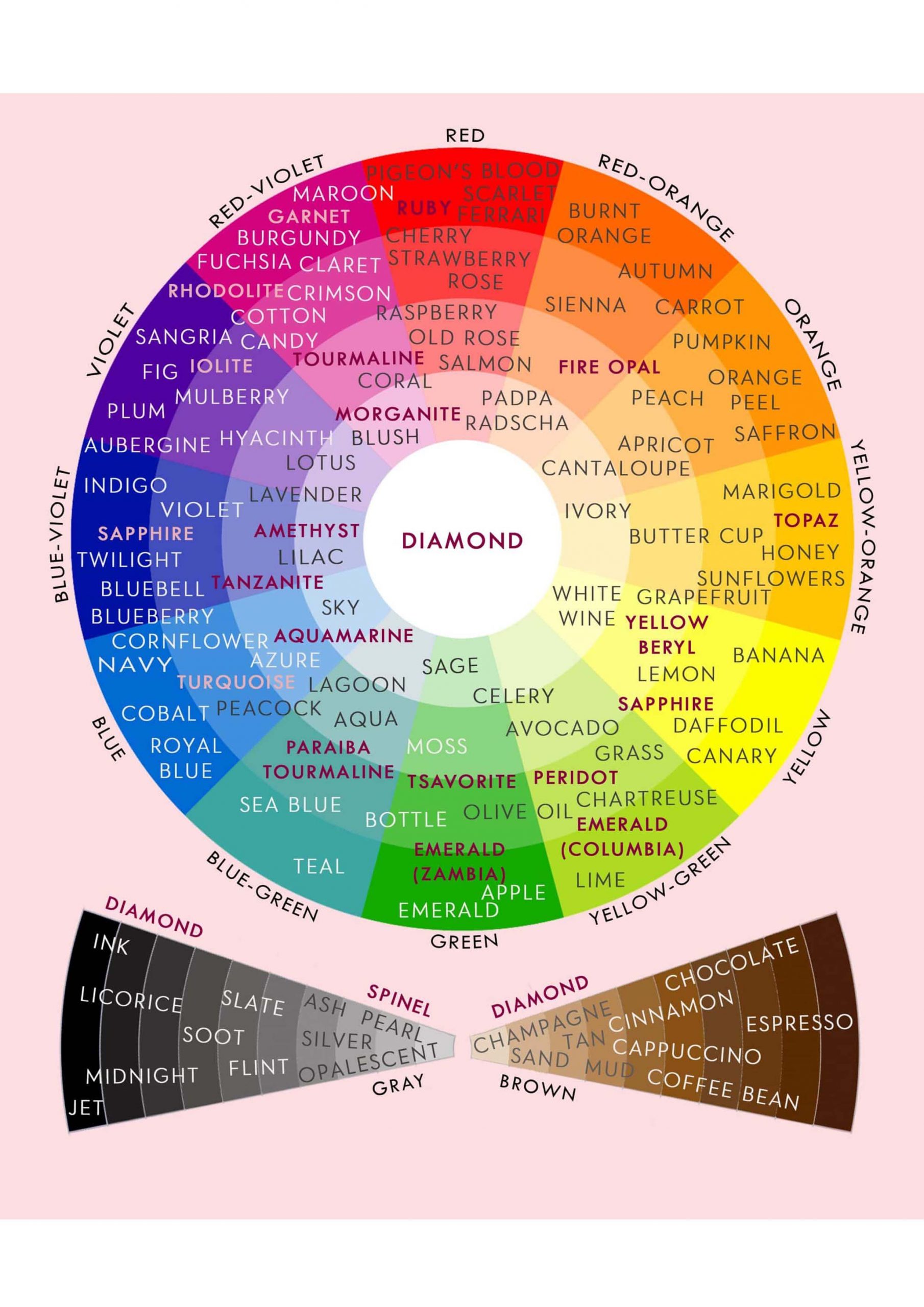
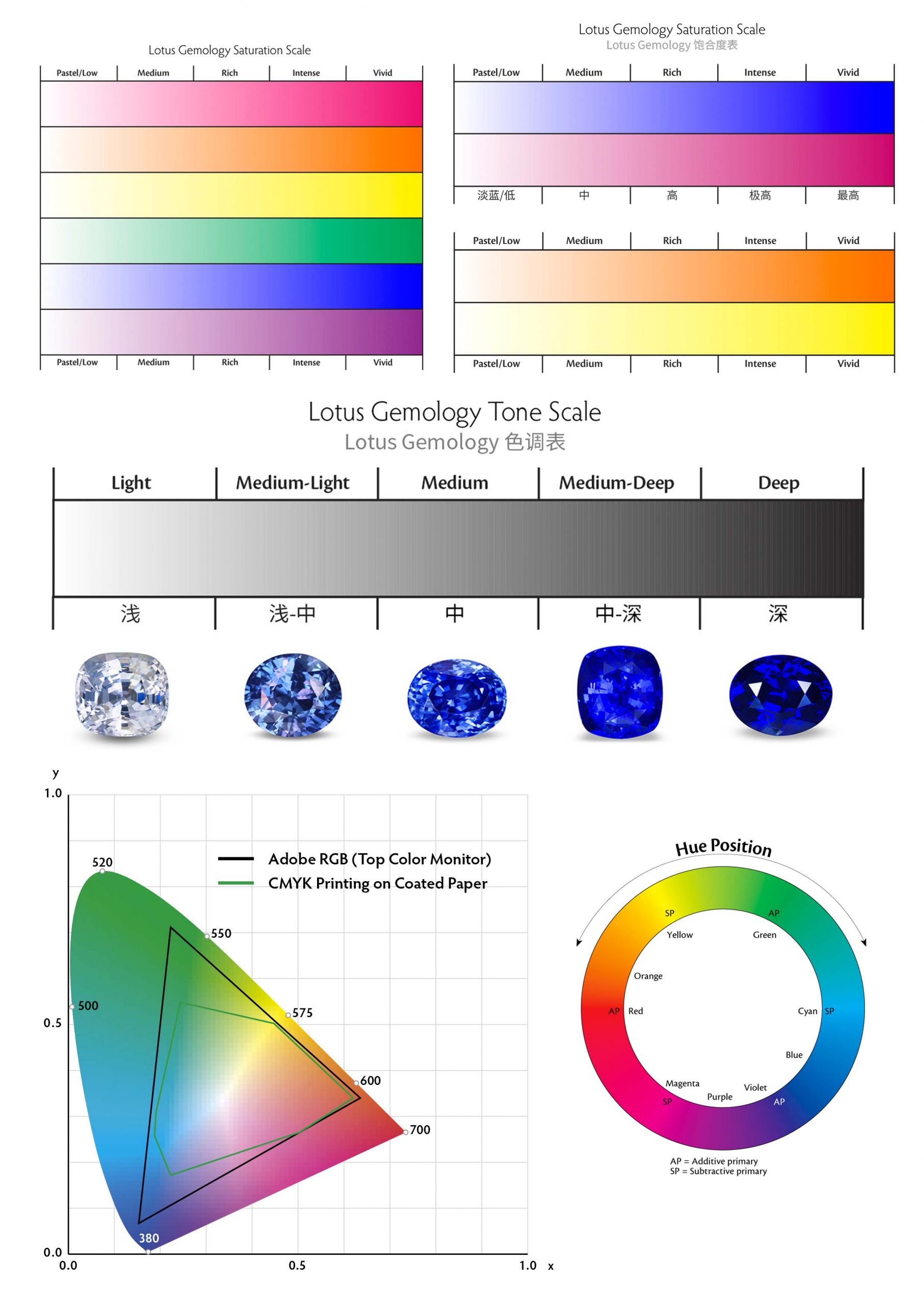
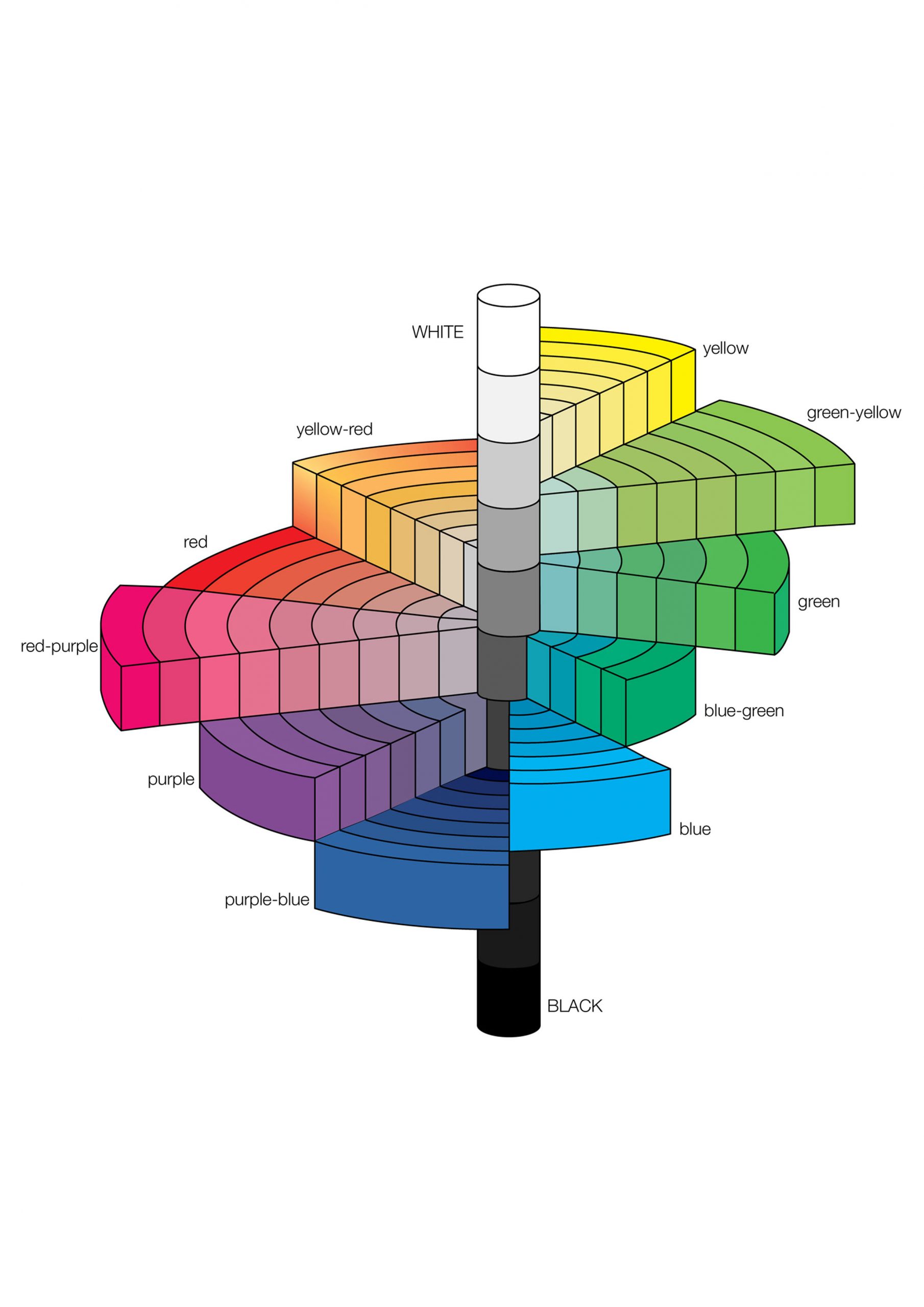
Gem color is made up of three elements: hue, tone, and saturation. To accurately define a gem’s color grade, all three factors must be considered.
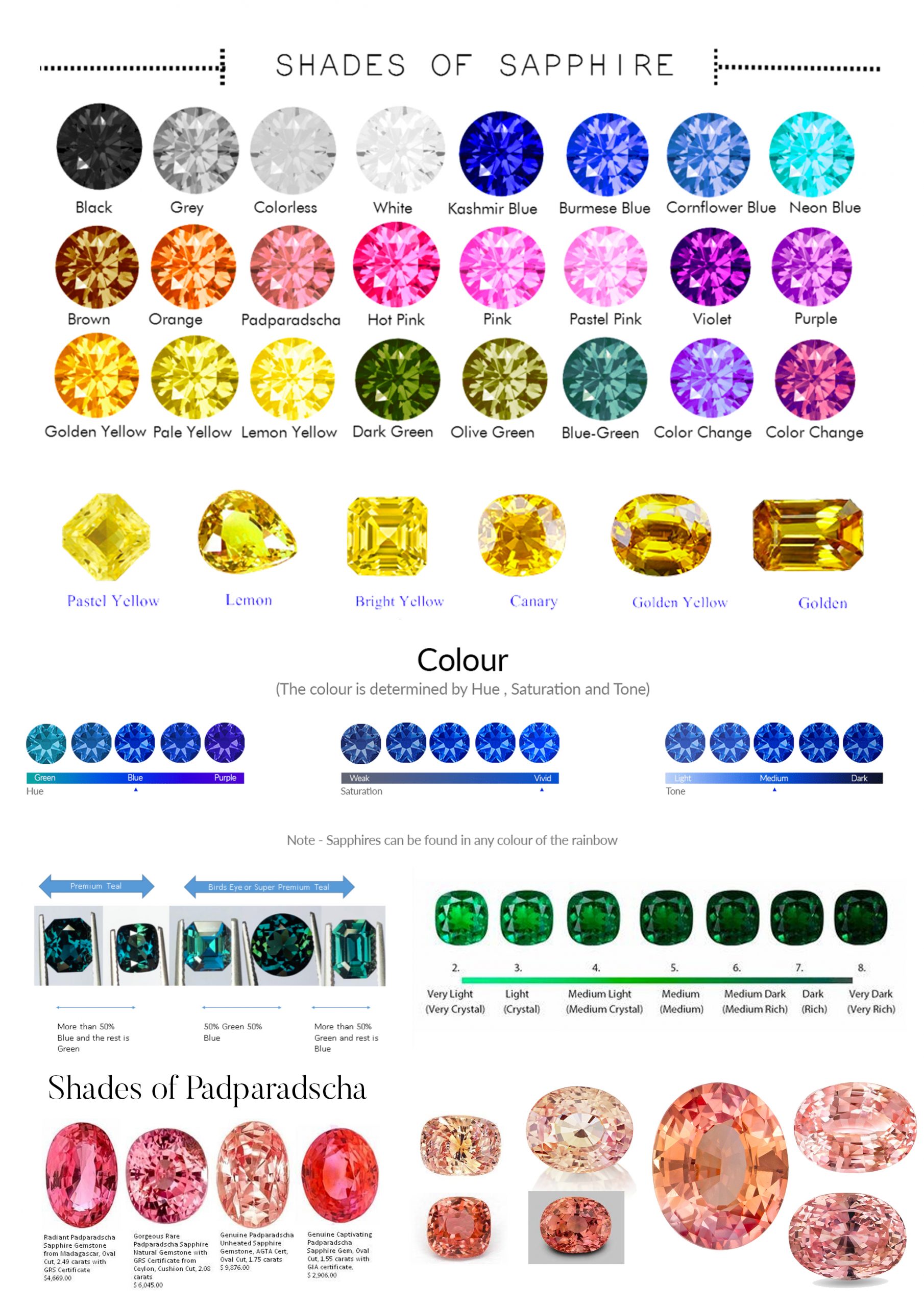
Hue
The term hue is the one we’re most familiar with. Red, orange, yellow, green, blue, violet and purple are the primary colors. Please notice that black, brown, and white are not included. While many people mistake these phrases for colors, they actually represent a lack of color rather than specific hues. (This is covered in the tone and saturation sections below.)
We define distinct colors as though they were compass points. Instead of referring to color as “SSE,” we describe it as “slightly yellowish orange.”
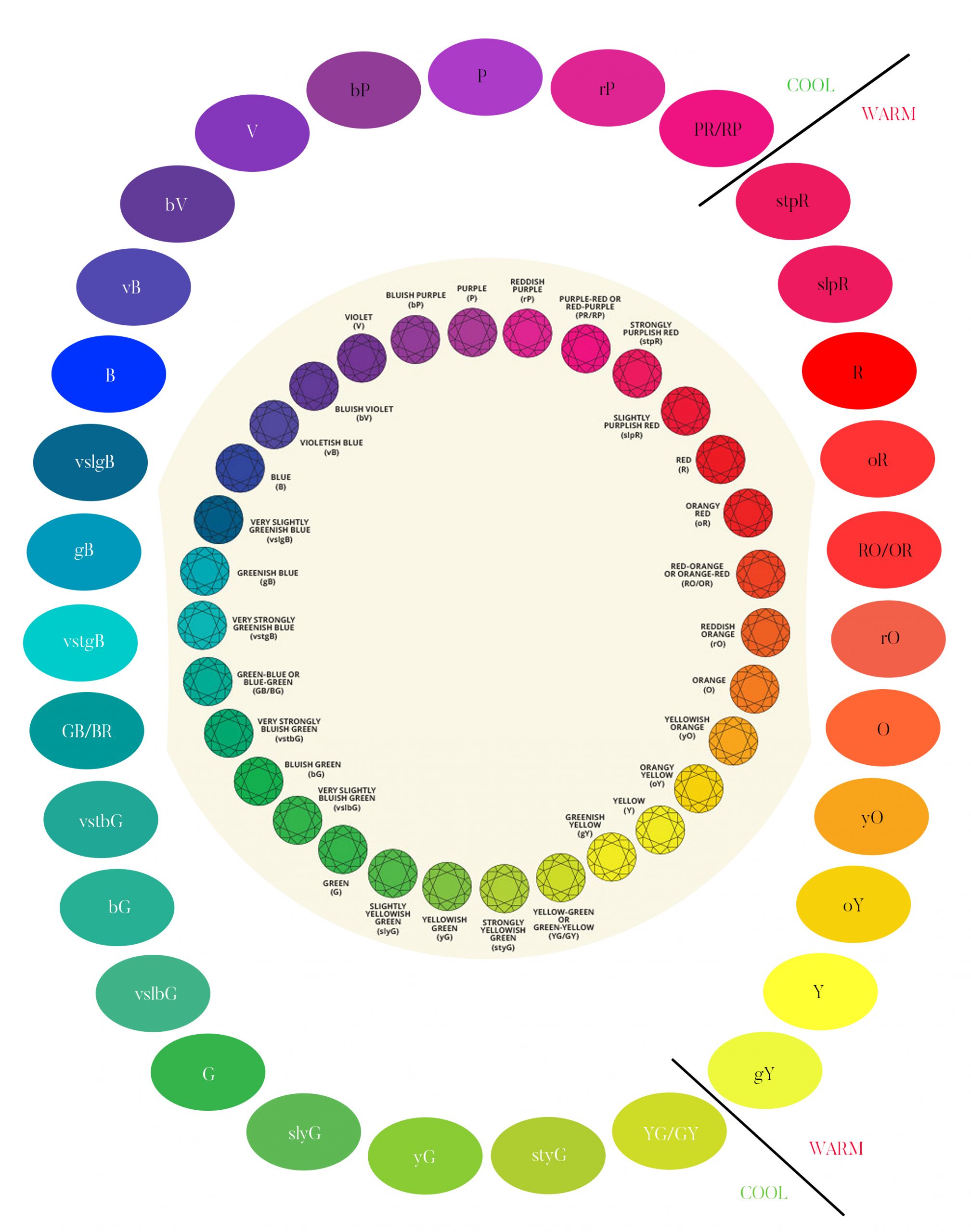
The primary color is always expressed last in this manner. It is preceded by descriptive phrases. Let’s start with the color yellow. It’s a pure color when written that way. We’d call it somewhat greenish Yellow if the color leans slightly towards green. If the green is more prominent, the color is described as a rich greenish-yellow. Green-Yellow or Yellow-Green is the color created when green and yellow are mixed evenly. (In an equal-opportunity situation, it doesn’t matter which color we list first.) We’d have intensely yellowish green, somewhat yellowish-green, and then pure green as we moved closer to the green. This pattern can be applied to any hue.
You’ll utilize a consistent approach for abbreviations and capitalization while drafting reports. The primary color should always be abbreviated and capitalized. All other letters should be written in lower case. The letters “sl” and “st” stand for “slightly” and “strong,” respectively. As a result, slvB denotes a somewhat violet Blue, while styO denotes a harsh yellowish Orange.
Tone

Tone refers to the relative lightness or blackness of a stone. We use the numerals 0 to 10 to express tone, which ranges from light to dark. 0 is colorless or white since it is so light. The number ten is so dark that it appears to be black. All stones have the same relative brightness/darkness grades.
The brightness of a faceted gem changes depending on the angle from which it is seen. When grading tone, looking down the table at the stone. It’s important to pay attention to this because it’s a regular procedure. When you look down on the table, you get the darkest view of any jewel. In other situations, it will be so black that the grade will appear incorrect. But keep in mind that we’re working with a standard. We must rate tone in all jewels in the same way for stability.
Extinction
 In extreme circumstances, the stone will turn entirely black when viewed from the front. The gem cutter made some poor crown facet angle selections, causing light to reflect back into the stone rather than escape through the crown. This is referred to as extinction. Extinct gems are the exception rather than the rule. To grade their tone, you’ll need to tilt them slightly. A rating of ten, on the other hand, denotes an opaque stone.
In extreme circumstances, the stone will turn entirely black when viewed from the front. The gem cutter made some poor crown facet angle selections, causing light to reflect back into the stone rather than escape through the crown. This is referred to as extinction. Extinct gems are the exception rather than the rule. To grade their tone, you’ll need to tilt them slightly. A rating of ten, on the other hand, denotes an opaque stone.
Saturation
The strength of a hue is referred to as saturation. Cool colors, such as blue and violet, become increasingly gray as their saturation decreases. As the saturation of warm colors like red, orange, and yellow drops, they become shades of brown. Black, brown, and white aren’t considered colors because of this.
There are six levels of color saturation. When discussing cool or warm colors, however, the descriptions differ slightly.
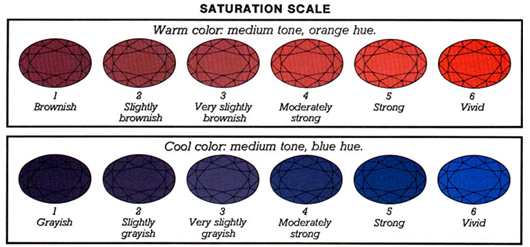
You must be able to tell the difference between tone and saturation. They’re two very different features. Higher saturation creates the impression of a lighter stone, as shown in the example above. All of the stones above, for example, have the same tone, which is 5 or medium. The ones with increased saturation, on the other hand, appear to be brighter.
Colored gemstones have different levels of saturation. The saturation of most gem species does not reach Vivid levels. This level is more likely to be found in outstanding rubies, sapphires, and paraiba tourmalines, however, it is rare. Emeralds have a maximum strength of five. Aquamarines have a maximum strength of 4 (Moderately Strong). When determining the value of jewels, keep this in mind. Rather of grading overall gem color, grade saturation per species. (Note that the term “Vivid” is also used to describe the color of fancy colored diamonds.)
Gem Values and Saturation
Understanding gem values requires full knowledge of saturation. The stones with the highest saturation levels receive the highest scores. The greater the effect of saturation on value, the more valuable the diamond. The prices of specific gem species are listed by color in our Gem Price Guide. The saturation of top-grade blue sapphires is 5 or 6. Sapphires of excellent quality have a saturation of 4.
While saturation is most noticeable in high-value gems, it has an impact on all of them. It’s the main reason why a carat of green tourmaline is worth $1,000 and another $200. The majority of tourmalines have low saturation. Only a few people make it to level 4 or 5. Africa has recently produced some really dark aquamarines. The majority of them have low saturation levels, which reduces their worth significantly.
Keep in mind that our color memory is terrible. While the difference between a blueish-green and a yellowish-green gem is easy to spot, tiny differences in saturation are difficult to spot without comparisons. Don’t rely on your recollection for hue when it comes to high-value stones. Carry comparison stones with you when you go shopping. This can be accomplished with some low-cost synthetics.
Examples of Gem Color Grading
Rubies have a maximum saturation of 6, whereas most gems have a maximum saturation of 4. The highest values are pure red or purple with a faint purple tinge. Rubies with a faint purplish tint have a higher value than rubies with an orange tint. Remember that your display won’t be able to recreate this amount of saturation, so the hue may be somewhat off. Only use these images for training purposes.
The color of the ruby in the image below is representative of a top Burma ruby. This color is known as “Pigeon Blood Red,” and it’s a somewhat purplish red. It has a powerful saturation of 6 and a medium-dark tone.
This ruby has a tone of 5 and a pure red color. Due to its softer tone, appraisers would deem it just shy of top color grade, despite the fact that it is a superb colored ruby.

This pink ruby is now even lighter in color than the prior jewels. It has the same saturation as the pure red ruby above, but a tone of 4 instead of 5. This will aid in the differentiation of tone and saturation.

The next two gems don’t have completely pure colors. As a result, their values plummet more quickly. When viewed separately, each appears attractive to the inexperienced. A seasoned professional would almost certainly notice a difference. The difference between them becomes apparent only when they are placed side by side with a pure red stone.
Creating an Appropriate Setting for Gem Color Grading
Colors in the Room
Keep the walls white or neutral hues when setting up a space to grade gem color. Obviously, the color you perceive will be affected by light reflecting off a colored wall. The same issue can be caused by light passing through a colorful curtain.
Eyewear
Wear colorless lenses if you wear glasses. Many people’s lenses darken as the light level rises. Low-cost alternatives include generic reading glasses and low-power magnifiers like the Optivisor.
Sources of Light
The single most crucial consideration is lighting. Natural, incandescent, and fluorescent lights all produce varying results when rating gem color. Organize your lighting such that you can only use one at a time. Even though it isn’t the major light source, a fluorescent ceiling light, for example, will have an impact on color. Set up your lights such that you can control them all from your grading table.
Light from the Sun
For gem color grading, you must have a window. Gems often change color or tone or show less dispersion when exposed to artificial light. You can’t accurately evaluate a gem if you don’t use natural light. You don’t have to grade everything by window light. You should, however, see if natural light makes a difference. Grading should ideally be done in a north-facing window. Any window can be used with the right shades. Most essential, examine your gem in natural, indirect light.
Because daylight is bright enough to overcome other sources, you usually don’t need to turn off your electric lights. If you have an electric light near the window, however, make sure it is shaded so it does not interfere with your grading.
Incandescent
While filtered sunlight is still the greatest light source, you can get by with incandescent light (either conventional or “daylight equivalent”) for most of your work.
Fluorescent
Fluorescent lighting is the least acceptable source since it trends heavily toward the yellow end of the spectrum. Under fluorescent light, a number of the stones you’ll likely study will have artificial color. This poses a severe difficulty for gem color grading. If your ceiling lights are fluorescent, turn them off when grading.
Please keep in mind that there are various different varieties of fluorescents available currently. Each has its own set of outcomes. Compact fluorescents, like soft white or “daylight equivalent” bulbs, do not have the same spectrum as regular long bulbs. If at all possible, avoid fluorescents except when looking for color change. Even yet, keep in mind that different types of bulbs may produce varied effects.
A Word on Color Change Phenomena in Gemstones
All of your gems must be checked for color changes. Make sure you have a range of light sources on hand for this.
Brightness
When it comes to color perception, brightness may make a big difference. A bright light will help you see the colors more vividly in dark stones. Too much light, on the other hand, will make it difficult to see the hue of a light-toned diamond. You can control the brightness of your light in two simple methods. To begin, slide the diamond closer to or away from the light. (This is the most straightforward technique.) Second, use a white towel or tissue to diffuse the light.
Procedures for Gem Color Grading
You’re ready to take your exam once you’ve prepared your grading area. Clean the stone before you begin.
Initial Examination
Hold the gem over a white surface with tweezers for a first inspection. Next, place the gem near a window and instantly shine an incandescent light on it. After that, place it close enough to the incandescent light (in the shade is optimal) that it is the only light shining on the gem. Make a note of any changes you see.
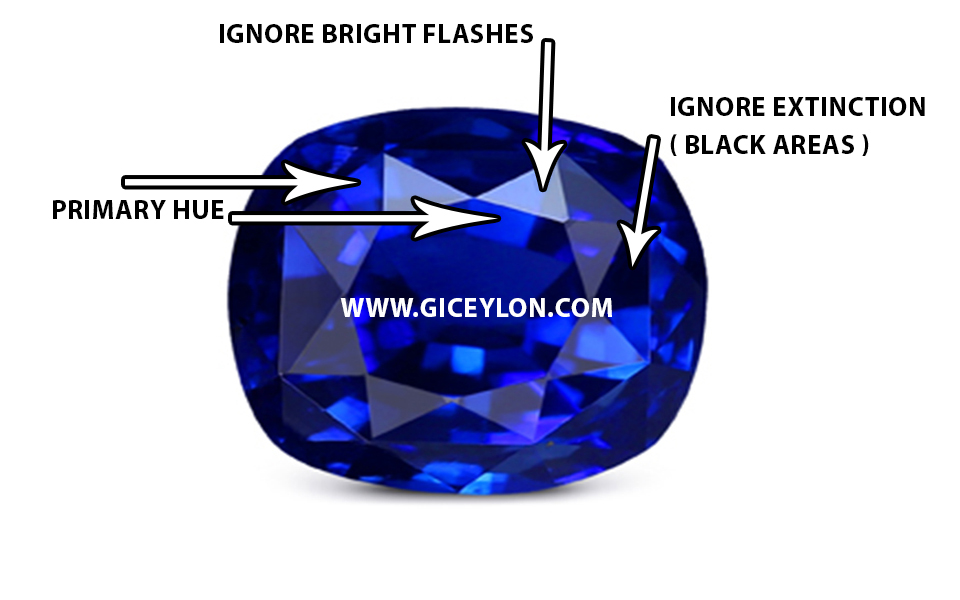
Describe the color scheme.
Begin by deciding on a primary color. Ignore any dispersion, as well as the brightest and darkest spots.
main hue – gem color
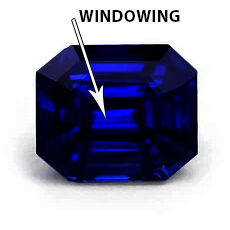
Dark stones might be difficult to work with at times. Tipping the stone until it windows is a good idea. The color of the “window” is usually your primary color. Nonetheless, examine the stone from the top to make sure.
windowed area – gem color
Be as specific as possible when describing the hue. Very little purplish-red isn’t considered “Red” in gem color grading. Grayish or brownish tones should be avoided at all costs.
This is a little tough at first, so compare with color masters. Your color masters can be low-cost synthetic gems, a GIA Gemset, or paint store color samples. Just make sure you’re comparing true hues.
Define the tone
After that, explain the tone. Remember to stare at the stone with your back to the table. Remember to look at master stones from the same angle if you’re using them.
Saturation should be described in detail.
After you’ve chosen the tone, look at the saturation of the stone. Think in terms of grayish or brownish to do this.
Remember that a darker stone seems lighter when the saturation is higher, and vice versa. So, when judging saturation, keep the tone in mind.
Creating a Color Description for Your Gem
The color should come first, followed by the tone, and then the saturation.
A rhodolite garnet, for example, might be described as stpR, 6/4. Strong purple Red, tone 6, and saturation level 4 are the results.
Don’t Put Your Trust in Illustrations
You can use the graphic from the section “The GIA Color Grading System” for grading tone.
However, don’t use the color and saturation illustrations in this article for grading. There are far too many variables in color reproduction to guarantee hue accuracy.
Worse, a computer monitor is incapable of reproducing high or vivid saturation levels. You’ll need comparison stones for this.
gemstone color grading methods are a bit different in some laboratories and they use their own systems in some places and countries.
gemstone color grading is critical and a must for gem traders and gemologists to win the trade
Gemstone Color Grading
Article Composed by :
සම්පත් සමරසේකර
Sampath Samarasekara
Chairman: Gemological Institute of Ceylon
Chairman: Youth Gem Professionals Association
Chairman: Sampath Gems
Director: Ceylon Sapphire Gems and Jewels
Director: Ceylon Gem Fair International
Chairman: Nanosoft Web Develop Company
Direct WhatsApp: https://wa.me/message/2A4AUALYVQWRA1
Join with Gemological Institute of Ceylon for Practical Gemology Studies
Facebook Page: https://www.facebook.com/giceylon
Facebook Group : https://www.facebook.com/groups/1990653001208203
Facebook Profile : GI Ceylon
Websites :
www.giceylon.com
www.bluesapphire.lk
www.gemluck.com
www.buyroughgems.com
www.ceylongemfair.com
Join with Gem and Jewellery Business Help and Discussion Group on Telegram :
Classifying Gemstones


Many gemstone names can be related back to ancient times before mineralogy provided a scientific basis for categorization.
Gemstones are identified in modern texts first by their mineralogical classification, then by the name of the mineral variety.
A radical is a group of atoms that act as a single unit.
Classifying minerals
Chemical composition and internal atomic structure are used to classify minerals. The chemical formulas for marcasite and pyrite are identical, but their interior structures are different.

Sulfides
A sulfide mineral is formed when a metal or semimetal combines with sulfur. In sphalerite, zinc is the metal.
Native Elements
Mineral formed from a single chemical element is called native elements. They include metals such as gold and nonmetals such as diamond, which is made up of carbon.
Oxides

Hydroxides
These minerals contain a hydroxyl ( Hydrogen and Oxygen ) radical combined with metal. In diaspore, aluminum is the metal.
Halides
A halide is a halogen element ( chlorine, bromine, fluorine, or iodine ) combined with a metal or semimetal. Fluorite is fluorine and calcium combined.
Carbonates
The carbonate radical, carbon, and oxygen combine with a metal or semimetal to form a carbonate. in the case of azurite copper is the metal.

Phosphates
In the phosphates radical of oxygen and phosphorus is combined with the metal or semimetal. Magnesium and aluminum are the combined medals in lazulite.
Borates
These minerals contain boron and oxygen. In howlite, boron, oxygen, and silicon combine with calcium and water.
Sulfates
Sulfur forms a radical with oxygen that combines with a metal or semimetal to form a mineral of the sulfate group, Barite is barium sulfate.
Silicates
In this group, silica (silica and oxygen) radical combines with various metals or semimetals. Silica also occurs uncombined as quartz, whose purple variety is amethyst.
Organics

Chemical formulas
The chemical makeup of a mineral is expressed in its chemical formula. the formula indicates the relative proportions of each chemical element that makes up a single structural unit of the mineral .some minerals grade into each other chemically, and this is also noted in the formula. minor amounts of other elements called trace elements, may also be present in a mineral and influence its color. however, these are not included in the formula.How gemstones are named?

 Message Gemological Institute of Ceylon on WhatsApp: https://wa.me/message/2A4AUALYVQWRA1
[learn_press_profile]
Message Gemological Institute of Ceylon on WhatsApp: https://wa.me/message/2A4AUALYVQWRA1
[learn_press_profile]
Gemstones formation
There are five requirements for crystals to form: ingredients and temperature, pressure, time, and space. To better explain the basics of mineral crystallization lets talk about rock candy for a second. Rock candy is simply crystallised sugar.
If you stir as much sugar as possible into a bottom of water you will see it begin to settle at the bottom of the pot. When no more sugar can dissolve, you have reached saturation point. The water can’t absorb any more sugar, this is known as super-saturated.
Now bring the pot to a boil- the saturation level changes at boiling point. You can add more sugar, you should do so until you reach super-saturation. At this stage, you should remove the pot from heat. As the water returns to room temperature the sugar it can hold returns to its previous level. The excess sugar comes out of its solution and crystallises as it does so.
Now, hang a string in the solution so the crystals have something to grow on. Ideally, weight the string to keep it straight. Once the water has totally cooled the string will be covered in crystals.
This is a great way to understand just how gemstones form.

Gemstone Formation Process
Generally speaking there are 4 ways gemstones can form. They are
- Igneous– These minerals are created deep within the earth (Diamonds, Ruby, Sapphire, Peridot)
- Hydrothermal– Similar to the rock candy example, gemstones are formed when bodies of mineral rich water cool
- Metamorphic– As the name suggests, these are gems that are ‘morphed’ due to intense heat and pressure. (Sapphire, Ruby, Spinel, Garnet)
- Sedimentary– Gems that form due to water depositing sediments (Malachite, Azurite, Opal)

Igneous Gemstones Formed in the Earth’s Mantle
While our knowledge of the earth’s mantle is limited, there is evidence that some gemstones form in the mantle. This requires extremely high temperatures.
Perhaps the most notable examples of gemstones forming in the earth’s mantle are peridot and diamond. Geologists studied Arizonan peridot deposits and believe that they were created on rocks that were floating in the earth’s mantle, up to 55 miles beneath the surface. They were brought closer to the surface by an explosive eruption, with erosion and weathering pushing them close enough to the surface to be discovered.
However, there is a better understanding of diamonds. Diamonds crystallise in the magma just below the crust. However, these formations have a different chemical composition. Geologists believe it comes from 110 miles to 150 miles beneath the earth’s surface. The magma is incredibly fluid at this depth, and the temperatures very high.

This magma can force its way through the crust far faster and much more violently than other volcanic eruptions. During the process of eruption, the magma breaks up and dissolves rocks and then carries them to the surface.
If the magma was to rise slowly the diamonds likely would not survive. The pressure and changing temperatures would result in the diamonds vaporising, or possibly re-crystallising as graphite. However, because of the speed with which the magma rises the diamonds don’t have time to transform or vaporise, thus remaining as diamonds.
When there are dramatic and rough changes in the crust crystals are often broken. When growth conditions are present material seeps into the fractures and crystallises. This heals the fractures by sealing them together. They don’t heal completely, though, the fine cavities remain and they are seen as fingerprints.
How do they rise to the surface once gemstones form? Because they form so far under the surface, it’s a wonder they can be mined. They’re brought to the surface during volcanic eruptions, but most of them reach the surface through erosion and mountain building.
Hydrothermal Gemstone Creation
This process is the most similar to the rock candy given above. Supersaturated water with many different minerals is pushed up into cavities and cracks in the earth. as this solution begins to cool the different minerals begin to crystallize.
The most important hydrothermal finds are in Columbia. Specifically the Muzo Emerald mine. These hydrothermal deposits are rich in Chromium with gives the Emeralds from the region their incredible color.
The image below shows a hydrothermal mineral vein. This vein is created when the water solution cools inside the crack of the surrounding rock.

Metamorphic Gemstone Creation
The majority of gemstones are formed by metamorphism. This is when minerals are forced together under great pressure and heat usually by tectonic plates moving underneath each other. The minerals are forced together and they metamorphose into different minerals, sometimes without melting.

Sedimentary Gemstone Creation
Sedimentary gemstone creation occurs when water mixes with minerals on the earth’s surface. The mineral-rich water seeps down between the cracks and cavities in the earth and deposits layers of minerals. This is how minerals like Opal, Malachite, and Azurite are formed. Opal is formed when the water mixes with silica. As the silica solution settles, microscopic spheres of silica stack on top of each other forming Opal.

Mineral Crystallisation
The earth’s crust can be anywhere from three miles thick to 25 miles thick. Beneath the crust is the earth’s mantle. The mantle is approximately 1,860 miles thick and makes up 83% of the earth’s volume. It is composed of magma, which is molten rock. When it reaches the surface, it is called lava. It is at its hottest nearer the center of the earth you get- and the currents of heat keep it in constant motion.
The zone where the crust and mantle meet is tumultuous, with high temperatures and high pressures. Several plates make up the earth’s crust and float on the liquid mantle. As they run into each other some are raised into mountains, while others are pushed down.
Magma is also in continual motion. Its pressure and movement are continuously creating wear and fractures to the bottom of the crust. Rocks then break free from the earth’s crust, being carried away by the fluid magma. The rocks melt, altering the magma’s chemistry. While the smaller particles are fated to become inclusions in gemstones yet to form.
Gemstones form deep in the earth, with the lower surface of its crust containing numerous cavities due to heavy fractures. Fluids escape through the cavities and fractures. This is the ideal condition for crystal growth. It is essentially a soup rich in chemicals, supplying all of the necessary ingredients. The cavities provide the perfect space to grow, and the pressure and temperature are high. The fluid moving through the crust causes it to cool enough for the crystallization to ensue- all that’s needed now is time.
Geologically speaking- the time it has should be sufficient. However, because this environment is highly tumultuous and the passages continuously open and collapse. The crystals will often start to form, but when the passage collapses the fluid stream is closed off. It’s at this point that growth stops.
If and when the passage reopens the growth resumes. This on/off growth is generally undetectable in crystals, though in other cases the successive layers of development have a different chemical composition. This results in color zoning.
Mineral Crystallisation Order
Topaz crystals form before quartz in the cooling process because one principle of crystallization is that as the temperature drops the solid ingredients it can hold drops. Though, the ingredients in the earth’s crust are a tad more complex than the sugar solution described above. Different minerals crystallize from the same solution, but at different temperatures. You may see corundum first, followed by topaz and quartz as the solution continues the cooling process.
While pressure has no effect on rock candy- the correct combination of temperature and pressure is needed for minerals to crystallize.
Additionally, there are two other conditions required for crystallization – space and time. Essentially, the correct ingredient combination, pressure, and heat must last long enough for minerals to crystallize. Additionally, they require room to grow.
…. Detailled Article
How Are Gemstones Created?
How to Create Your Own Crystals?

Making crystals is an excellent way to learn about crystal growth. To make rock candy, the simplest method is to crystallize the sugar.
- Add as much sugar as possible to a pot of water. When it reaches the bottom and no longer dissolves, you have reached the saturation point. The water has absorbed all of the available sugar. This is referred to as super-saturation.
- Following that, bring the pot to a boil. When water boils, the saturation point changes. The solution is no longer super-saturated. You can now significantly increase the sugar content. Therefore, continue adding sugar until you reach a super-saturation level.
- Take the pot off the heat. When the water reaches room temperature, the amount of sugar suspended in it returns to its previous level. Excess sugar must be removed from the solution. It will crystallize as it does so.
- In the sugar solution, hang a string for the crystals to grow on. (At the bottom of the string, fix it with a weight to keep it straight.) Although the process is too slow to observe without assistance, you will notice changes in the crystals every few minutes.
- When the solution reaches room temperature, the string will be completely covered in sugar crystals. Again, the water will be supersaturated.
The Fundamentals of Mineral Crystallization
This straightforward exercise introduces you to four of the five conditions necessary for mineral crystallization to occur.
- Ingredients
- Temperature
- Pressure
- Time
- Space
Crystals are composed of more complex and numerous ingredients than our sugar solution. Various minerals may be present in solutions.
At a greater temperature, a solution can suspend a large number of minerals. As the temperature drops, the amount of suspended solids decreases as well. Crystals form as a result of this. Indeed, different minerals crystallize at different temperatures in the same solution. Corundum, for example, may crystallize first. As the solution cools further, topaz and then quartz may form.
There is no effect of pressure on the formation of rock candy. However, minerals crystallize only under the right conditions of pressure and temperature. Underground crystallization of gems typically requires extremely high pressures and temperatures.
Time and space are relatively simple requirements. The optimal combination of ingredients, heat, and pressure must exist for an extended period of time in order for the minerals to crystallize. Additionally, they require room to grow. Obviously, a crystal 5 cm in length cannot grow in a cavity only 5 mm in diameter.
Underground Environment
Consider the underground conditions that permit crystallization and the formation of gems.
The thickness of the Earth’s crust varies from 3 miles (around 4.8 kilometers) beneath the seabed to 25 miles (around 40 kilometers) beneath continents.
The mantle is approximately 1,860 miles (around 2993 kilometers) thick beneath the crust and accounts for 83% of the Earth’s volume.
It is made up of molten rock known as magma. When it does reach the surface, we refer to it as lava.
Near the Earth’s center, the mantle is hottest, and heat currents keep it in constant motion.
The crust and mantle cross paths in a violent zone characterized by high pressures and temperatures.
The crust is composed of several plates that float on the liquid mantle. As they collide, some are pushed down and others are elevated into mountains.
Additionally, the magma is in constant motion. Its constant movement and pressure act on the crust’s bottom, causing wear and fracturing.
Its constant movement and pressure act on the crust’s bottom, causing wear and fracturing. As a result, rocks separate from the crust and are carried away by the magma’s fluidity.
Much of this rock material melts, altering the chemical composition of the magma nearby.
Several of the smaller particles will be used as inclusions in future gemstones.
The lower surface of the crust is heavily fractured and contains numerous cavities.
The magma’s fluids flow through these fractures and cavities. Here, the ideal conditions for crystal growth exist.
Chemically rich fluids provide the necessary components. Cavities provide space for growth.
The temperature and pressure are extremely high in this area. The fluid cools sufficiently as it passes through the crust, allowing crystallization to occur. The only remaining requirement is time.
How Crystal Growth Interruptions Affect Gem Formation?
You might believe that time is more than sufficient for crystal formation in geological terms.
However, passages constantly open and close in this highly turbulent environment.
Often, crystals begin to form and then the passageway feeding the cavity with mineral-rich fluid closes.
At this point, no further growth occurs.
Growth will resume if the passage is reopened.
Growth will resume if the passage is reopened. This on-and-off growth pattern is usually undetectable in crystals. However, it does have noticeable effects in some instances.
Zoning by Color
Occasionally, the chemical compositions of successive layers of growth will be slightly different. When this occurs, the crystal may exhibit color zoning.
Parting
In some twinned crystals, the new layers do not completely bond to one another. When you see parting on a star ruby, for example, this indicates that the layers did not bond properly.
Crystallized Mineral Specimens
Even if a closed passage is reopened and fluid is allowed to enter a cavity again, a completely different mineral may crystallize over the previously crystallized material. Indeed, the temperature, pressure, and chemical composition of a fluid solution frequently change over time. Within a cavity, different conditions will result in the formation of different mineral crystals. When you open a deposit, you will frequently notice that different minerals cover earlier layers.
Inclusions
Such changes in the internal conditions of a cavity are also one of the causes of gemstone inclusions. A new crystal may begin to grow on the surface of an older, larger one, only to have its growth stopped. If the original crystal-growing conditions are restored, the older crystal will grow over the newer one.
Occasionally, two distinct minerals crystallize simultaneously. If one takes off and begins to grow at a faster rate, it will eventually entrap the other. That is how pyrite crystals become embedded in emeralds.
Chemical impurities can also exist within a crystal under certain conditions. When the temperature and/or pressure of the host crystal change, the impurities can crystallize within it. (In effect, the host crystal serves as a cavity that contains ingredients that require only the right conditions to crystallize.) This is the process by which rutile forms within quartz and corundum.
Phantoms
Phantom crystals or inclusions may form in rare instances. This occurs when a new layer of crystals forms on top of an existing transparent crystal. A fine layer of feldspar, for example, may cover a quartz crystal. Subsequently, conditions revert to their original state, and the original transparent crystal resumes growth. This time, the feldspar is covered by a new layer of quartz. The resulting gemstone exhibits an indistinct outline of that fine secondary crystal layer, resembling a nearly transparent ghost or phantom, hence the name.
Heling Fractures
Numerous crystals are broken during the rough and dramatic changes in the crust. However, if growth conditions exist, material may seep into fractures and crystallize. This effectively “heals” the fracture by regrowing the crystal. However, these fractures never completely heal. In the previous gap, fine cavities filled with gas remain. This type of inclusion is referred to as a healing fracture. Due to the similarity between the fine cavities that remain and fingerprints, gemologists also refer to these inclusions as “fingerprints.”
Strain
Numerous crystals are compressed beyond their natural size due to the tremendous pressures present in the underground environment of gem formation. Additionally, this strain can make a stone more brittle. This level of strain may be present in tourmalines, garnets, and even diamonds. Numerous faceters have shattered these stones when they were placed on a lap. The forces within the stone literally explode it.
Geological Processes and the Formation of Gemstones
Gemologists now have a good understanding of mineral crystallization. Advances in geology and synthetic gem manufacturing have aided in the unraveling of several of nature’s mysteries. (Creating gems in laboratories involves simulating underground conditions, but significantly reducing the time required.)
Traditionally, we were taught that there are three distinct processes that result in the formation of rocks:
Igneous rocks are formed by intense heat deep in the Earth.
Metamorphic rocks are formed when existing minerals undergo transformation due to heat and pressure.
Sedimentary rocks are formed when sediment is deposited.
Geologists now prefer to describe rock formation in terms of four distinct processes:
Molten rock and fluids associated with it
Changes in the environment
Water on the surface
Formation of gems in the Earth’s mantle
We will look at how each of these processes contributes to the formation of gems.
Regardless of our knowledge, mineral and gemstone formation are neither simple nor straightforward. Minerals and gems are constantly destroyed and recreated as a result of the “Rock Cycle,” as illustrated in the chart below.
Molten Rock and Associated Fluids
Gems rarely form in the Earth’s magma. Rather than that, they form as a result of fluids escaping from it, as with gems from hydrothermal deposits and pegmatites. To begin, we’ll discuss two notable exceptions to this rule: magma and gas crystallization.
Crystallization of Magma
Magma is composed of numerous elements. The elements combine to form minerals as it cools. Which mineral is used depends on the available ingredients, the temperature, and the pressure. Each time a mineral crystallizes, the ingredients that are available change (since some ingredients are pulled into crystals). Different minerals form as magma progresses through various stages of changing temperature, pressure, and chemistry.
Aggregates
However, crystals will not form unless the conditions are ideal. Rather than that, magma will simply cool into a solid mass of small, interlocking crystals referred to as an aggregate by gemologists.
Phenocrysts
In some instances, a single mineral crystallizes beautifully. The magma will then seek out a crack in the crust and rush to the surface, destroying any remaining crystals. The pressure and temperature are too low for crystallization to occur. Rather than that, the remaining magma cools into fine-grained rocks, leaving the original crystals scattered throughout the interior. These are referred to as phenocrysts.
Phenocrysts of corundum, moonstone, garnet, and zircon are frequently discovered. Thailand’s Chanthaburi and Trat districts are rich in ruby and sapphire phenocryst deposits.
The Crystallization of Diamond
Diamonds crystallize at significantly higher temperatures than other minerals. Scientists now believe that the majority of diamonds form in magma, close to the Earth’s crust, where the temperature is the coolest. If this is true, it also means that the most favorable conditions for diamond crystallization exist underground. Diamonds are possibly the most abundant crystals on the planet. They are simply not the most accessible.
Crystallization of Gas
Have you ever wondered why some crystals are doubly-terminated, while the majority of crystals are broken at the base? The majority of crystals form on a solid matrix of other minerals. However, a few thrive within gas bubbles! After the magma reaches the surface, these gems form. During a volcanic eruption, rising magma’s pressure rapidly decreases. This results in the formation of gas bubbles, much like when removing the cork from a bottle of champagne.
Occasionally, these bubbles will contain elevated concentrations of particular elements. If the proper temperature and pressure conditions exist for an extended period of time, doubly terminated crystals form.
The so-called “Herkimer diamonds” are some of the most well-known examples of gas crystallization. The first half of the name of these quartz crystals comes from their source in Herkimer, New York. To some gem enthusiasts, these gems’ water-clear clarity and shape evoke the appearance of diamonds, hence the second half of their nickname. (Obviously, quartz gemstones are not diamonds.)
Garnet, topaz, and spinel can also crystallize as a result of gas crystallization.
Deposits of Hydrothermal
Hydrothermal crystallization, as the name implies, involves the use of water and heat. Water dissolves minerals as it percolates through the Earth. (Exactly as the sugar in your rock candy did). It comes into contact with magma deep within the Earth. The magma then spews out special fluids containing water, carbon dioxide, and volatiles (substances that give off the gas).
These hydrothermal fluids flow through crustal fractures. They may dissolve minerals or combine with other groundwaters along the way. Mineral-rich fluids cool in “veins.” Crystals form when the right conditions of temperature, pressure, time, and space are met.
Hydrothermal deposits are unique in that they may contain elemental combinations not found elsewhere. The Muzo emerald field in Colombia is one of the most significant hydrothermal gemstone deposits.
Pegmatites
At times, the magma in the upper mantle becomes concentrated with volatiles. Occasionally, this volatile-rich magma is forced into a cavity, where it cools and crystallizes into a pegmatite. Pegmatites are distinct from hydrothermal veins in that they are formed by magma rather than water.
Changes in the Environment
Within the Earth, extremely large pressure exists. Temperatures and pressures can rise to a point where existing minerals cannot remain stable under the right conditions. This can result in the transformation of minerals into new species without melting. This is referred to as metamorphism.
Metamorphism is classified into two types: contact and regional.
Make Contact with Metamorphism
When magma forces its way into an existing rock formation, this is called contact metamorphism. Existing rocks begin to melt and eventually recrystallize as new species when exposed to extreme heat. These are thermally stable at elevated temperatures.
Regional Metamorphism
Regional metamorphism is much more widespread than contact metamorphism. It has a much broader range of mineral effects.
The Earth’s surface is made up of large pieces called “continental plates.” They float on the mantle and are constantly moving on a geological time scale. They do not, however, all move in the same direction. Several of them are even in direct competition for the same space. Where these massive structures are pressed together, one is pushed beneath the other, while the other is pushed upward. This is the primary method of mountain formation on our planet.
Where these landmasses meet, enormous compression forces exist. This results in the formation of an area of extreme heat and pressure. When the temperature in the area approaches the melting point of the rock, the minerals become unstable. They evolve into new species over time (possibly millions of years).
East Africa is a prime example of a region undergoing regional metamorphism. Certain minerals discovered here are unique, such as tanzanite and commercial-quality tsavorite.
The Development of Mineral Species
To comprehend some of the changes that occur to minerals during metamorphism, it is necessary to understand what constitutes a mineral species: both its chemical formula and crystal structure. Occasionally, during metamorphism, a mineral change only one of these properties. This is still considered a species change. These changes result in the formation of polymorphic and pseudomorphic species.
Polymorphs
Polymorphs are minerals that share the same chemistry but have distinct crystal structures. Mineral pairs that are polymorphous are occasionally referred to as dimorphs or dimorphous.
For instance, andalusite, kyanite, and sillimanite are all composed of the same chemical compound, Al2SiO5. They frequently polymorph into one another during metamorphism, when their chemical constituents recrystallize into new crystal structures. As a result, they evolve into distinct but polymorphous species.
Pseudomorphs
Pseudomorphs are minerals that undergo chemical transformations without altering their external crystal structure.
While some minerals undergo metamorphism, they retain their characteristic crystal structures and exhibit no abnormal properties. However, a crystal’s chemistry can change without recrystallization. Pseudomorphs are these one-of-a-kind minerals. A pseudomorph is an atomic-by-atomic substitution of one mineral for another without altering the outward shape of the original mineral.
A perfect example of a pseudomorph is the tiger’s eye. Quartz has replaced the original crocidolite (a type of asbestos) in these gems, but they retain the crocidolite’s fibrous structure.
Marcasite can pseudomorph into other minerals such as pyrite, gypsum, fluorite, and goethite.
Azurite frequently pseudomorphs into malachite, resulting in an azurite crystal with the perfect malachite crystal shape.
When a mineral forms a pseudomorph, it is referred to as the “after” mineral. Thus, we may encounter “pyrite following marcasite,” “gypsum following marcasite,” “malachite following azurite,” and so forth.
Water on the Surface
Rain is critical for mineral recycling. Erosion erodes rocks and repositions them. Once on the ground, rainwater plays a critical role in the formation of new gems.
Formation of Fossils
Water picks up chemicals as it travels through the Earth, transforming it into a weak acid. When heated or when combined with the appropriate chemicals, it can become extremely corrosive. This increases the ability of water to dissolve additional minerals. As water percolates through the Earth, it picks up a variety of substances. When it becomes too saturated to carry any additional material, it deposits the excess in the cracks and pores of existing rocks. This is the process by which fossils and petrified wood are formed.
In other circumstances, water comes into contact with mineral combinations that initiate a chemical reaction. The dissolved minerals are then deposited in seams and cavities as new minerals. Opal, turquoise, azurite, and malachite are all formed in this manner.
Formation of Opals
Much of central Australia was covered by an inland sea during the Cretaceous period. When it dried, it left a layer of silica-rich sands in the area. Rain has been dissolving silica for millions of years. Summers are hot and dry, and the groundwater evaporates to the point where the remaining water is unable to suspend the silica. Excess moisture is deposited in seams and cavities near the surface. Opal is a silica deposit.
Waterborne Minerals Can Create Gem Color
Certain gemstones receive their color primarily from minerals that are necessary for their chemical composition. Turquoise, azurite, and malachite, for example, all receive their color from copper dissolved in water. To form azurite or malachite, copper-rich water must pass through limestone. Turquoise also requires some phosphorous to be added to the water along the way.
Gems Formed in the Mantle of the Earth
Our understanding of the Earth’s mantle remains rather limited. However, evidence indicates that certain gems do form in the mantle. They must crystallize at an extremely high temperature in order to do so. Diamond and peridot are two of the most well-known gems formed in the Earth’s mantle. (Intriguingly, both of these gems can be found extraterrestrially. Both of these elements have been discovered in meteorites).
Formation of the Peridot
Geologists now believe that some peridots were formed on rocks floating in the mantle, approximately 20 to 55 miles (32 to 89 kilometers) below the surface. They were brought close to the Earth’s surface by an explosive eruption. Weathering and erosion eventually brought them to the surface, where they were discovered.
Formation of a Diamond
The formation of diamonds is now better understood. As previously stated, the majority of diamonds crystallize in the magma beneath the crust. However, the magma formations in which they are found are chemically distinct. They may originate from greater depths, approximately 110 to 150 miles (177 to 241 kilometers) below the surface. Temperatures are higher and the magma is very fluid at this depth.
This hot and fluid magma can pierce the crust more quickly and violently than other types of volcanic eruptions. It will disintegrate and dissolve lower mantle rocks during this process and then transport them to the surface.
If the magma rose more slowly, the diamonds would almost certainly perish. They would vaporize or recrystallize as graphite as a result of the changing temperatures and pressures. The magma may rise at such a rapid rate that the diamonds do not have time to transform.
How Mountain Formation and Erosion Bring Gemstones to the Surface?
As previously stated, a few types of gems are exposed during volcanic eruptions. What about the countless others that crystallize deep underground? The majority of these gems reach the surface as a result of mountain formation and erosion. Mountains rise as a result of the movement of the continental plates over long periods of time. The mountain is then weathered away over time, leaving the gem deposits near the surface. Naturally, this process takes hundreds of millions of years.
Article Composed by :
සම්පත් සමරසේකර
Sampath Samarasekara
Chairman: Gemological Institute of Ceylon
Chairman: Youth Gem Professionals Association
Chairman: Sampath Gems
Director: Ceylon Sapphire Gems and Jewels
Chairman: Nanosoft Web Develop Company
Direct WhatsApp: https://wa.me/message/2A4AUALYVQWRA1
Join with Gemological Institute of Ceylon for Practical Gemology Studies
Facebook Page: https://www.facebook.com/giceylon
Facebook Group : https://www.facebook.com/groups/1990653001208203
Facebook Profile : GI Ceylon
Websites :
www.giceylon.com
www.bluesapphire.lk
www.gemluck.com
Join with Gem and Jewellery Business Help and Discussion Group on Telegram :
https://t.me/joinchat/Q5cLnxilNw4xAr8T3TumfA
All images and text content are Copyrighted ©️
[learn_press_profile]


 ‘
‘