Education
Gemstones Inclusions
Gemstones Inclusions
By Phase Filing
Phase – Some of the substances with identical characteristics of physical state, chemical composition, and physical properties. Ex: two immiscible liquids inside the inclusion are described as two different phases, despite being both presented within the liquid state.Single-phase inclusions

Solid, Liquid, or Gaseous
Solid particles frequently contain single-phase inclusions (inclusions of other minerals or glass such as solidified melt from crystallization medium). Gaseous single-phase inclusions are found in a wide variety of natural and manmade glasses, as well as synthetic materials developed from a melt. Single-phase liquid inclusions are uncommon since fluid entrapment in a cavity is often caused by high pressure and temperature conditions. And when originally homogenous fluid is cooled to room temperature, it separates into two phases – liquid and gaseous – generating the relatively typical two-phase liquid-vapor inclusions.Single-phase solid inclusions
This type of inclusion may be extremely widespread in nature. Such inclusions can be seen in the form of rutile or tourmaline needles in quartz, pyrite, or calcite crystals in emerald, zircon, or apatite in sapphire, olivine or garnet in diamond, and so on. They have simply captured crystals of other minerals within the host crystal. They can form prior to, along with, or after the crystallization of the host mineral. Occasionally, such inclusions are properly formed and represent a collectible object. They will be common or uncommon, and they will provide color or special optical phenomena to the stones. We can mention instances where multiple different minerals are stuck together as inclusions within a crystal. A crystal forming in a metamorphic deposit, for example, may trap a portion of the host rock composed of multiple minerals. Each mineral corresponds to a different solid phase in such circumstances, and hence such inclusions cannot be regarded as single-phase solid inclusions. They are classified as two-phase, three-phase, or multi-phase inclusions, depending on the number of different minerals comprising the aggregate.
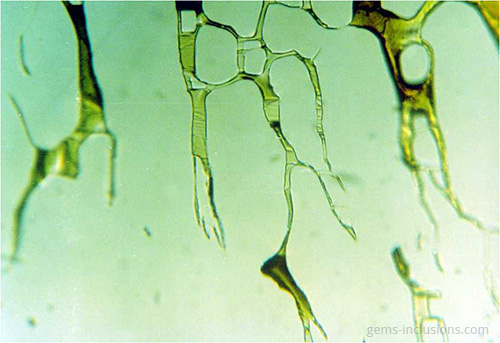
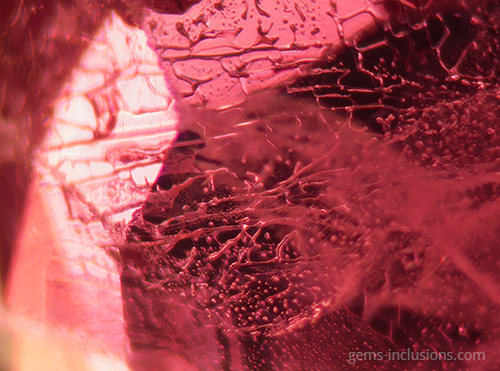
Single-phase liquid inclusions
Liquid inclusions in a single phase are uncommon. While many minerals crystallize from homogeneous aqueous fluids, the temperature at which they form is significantly higher than the temperature at the Earth’s surface. Thus, primary homogeneous fluid typically separates into at least two phases – liquid and gaseous – resulting in the formation of water filled with a gas bubble. Inclusions of liquid are frequently observed in crystals formed at low temperatures and pressures in shallow deposits. Additionally, they can form as a result of the separation of larger inclusions due to recrystallization of the cavity’s interior surface, a phenomenon known as “necking down.” As a result, the gas bubble is frequently contained within only one of the resulting separated cavities, with the rest containing only the liquid phase. In studies of fluid inclusions, inclusions that have been necked down are unsuitable for microthermometric analysis because their thermodynamic properties no longer correspond to the primary entrapped fluid.
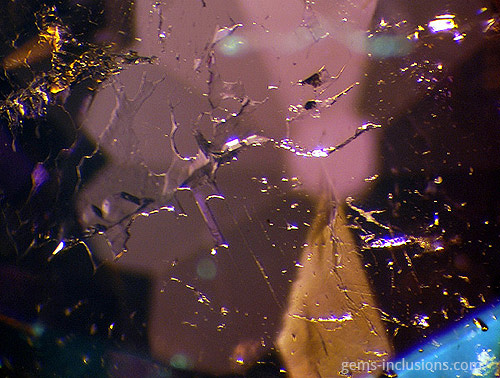
Single-phase gaseous inclusions
These are gas bubbles that are frequently observed in glasses or crystalline materials formed when melted substances solidify. In this case, the medium for solidification is gaseous, as are the inclusions trapped during the process. Gaseous inclusions have spherical and elongated rounded shapes. Gaseous inclusions are frequently found in natural glasses such as obsidian, moldavite, and Libyan glass. They are also characteristic of man-made materials such as synthetic glass and any melt-grown synthetic analogs of natural gems such as Verneuil ruby, sapphire, and others. The gaseous medium disallows the material from recrystallizing at the cavity borders, resulting in the formation of negative crystals or the necking down phenomenon characteristic of fluid inclusions. Rather than that, trapped gas bubbles remain perfectly round, making them easily recognizable. They can exist as isolated spheres or as galaxies of bubbles of varying sizes that form groups, clouds, or veils. Gaseous bubbles are also frequently observed in the rubies and sapphires filled with lead glass that have flooded the market since 2004. These stones, which gemologists currently classify as synthetic, are largely composed of lead glass that fills large fractures and cavities in very low-quality natural corundum. The glass is typically populated bubbled, which provides an easy way to identify such stones, in addition to a noticeable flash effect observed on the filled fractures. Gas bubbles are frequently visible in natural resins that have solidified to form copal or amber. Hardening occurs in this case not as a result of the transition from melt to solid-state via cooling, but as a result of the polymerization of natural resins over time, heat, and pressure produced by overlying sediments. Certain bubbles in amber may also contain a liquid phase, most likely water captured in the resin deposition process.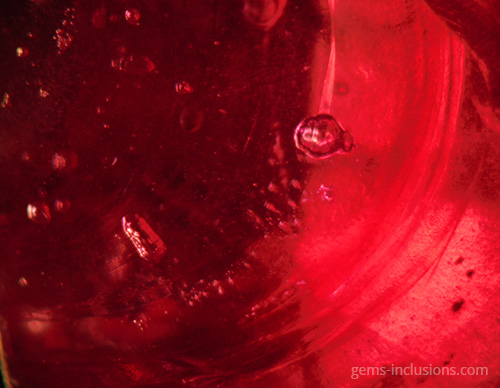

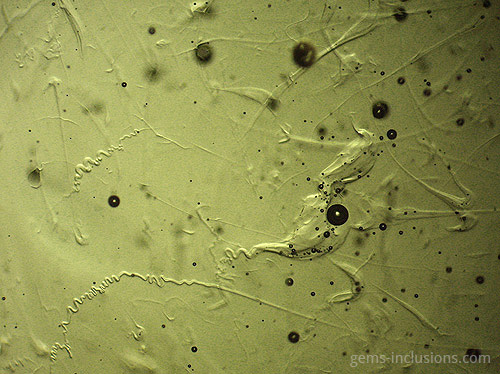

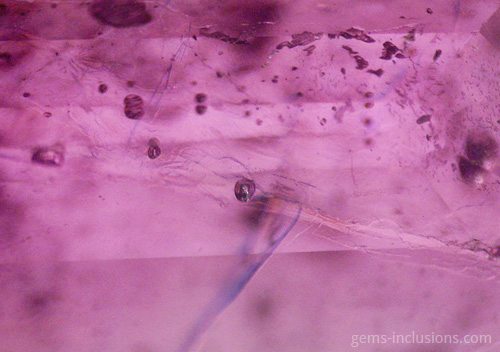
Two-phase inclusions
Cavities within crystals filled with two distinct phases are referred to as two-phase inclusions. Although liquid-vapor inclusions are the most common type, other combinations such as two immiscible liquids such as water and liquid CO2 or water and liquid hydrocarbons (petroleum) are also possible. It is critical to emphasize that the majority of two-phase inclusions, as well as three- and multi-phase inclusions, were trapped during the crystallization process as a single-phase fluid that later separated into two or more phases upon cooling to room temperature. Additionally, solid phases are frequently caused by solution, resulting in the formation of a crystal of a so-called “daughter mineral” within the cavity. In general, all phases that are distinct from a primary homogeneous fluid trapped during crystal growth are referred to as “daughter phases.” These phases include liquid, solid, and gaseous phases that form inside the inclusion cavity as a result of cooling to observation conditions. When the fluid inclusion is heated, all daughter phases gradually fade away, restoring the original homogeneous fluid. The following sections describe various types of two-phase inclusions. Liquid-vapor inclusions in two phases This is, without a doubt, the most common type of fluid inclusion, occurring in a wide variety of minerals and gems. Numerous crystals are formed from aqueous fluids at temperatures much higher than those at which we observe them. When the sample is heated to what is known as the homogenization temperature, we observe only one phase, which corresponds to the primary fluid. The temperature of homogenization is proportional to the lowest temperature at which crystals form, providing valuable information for mineralogical studies. Not every two-phase inclusion begins as a homogeneous fluid. When natural boiling occurs during mineralization, liquid and vapor phases may coexist within the primary fluid. One frequent scenario that results in boiling is the opening of closed fractures and the resulting decrease in hydrostatic pressure within the system, which results in boiling without the need for additional heating. Additionally, decreasing pressure can result in faster crystal growth (and entrapment of various inclusions) because the solubility of numerous minerals increases as pressure decreases. These two-phase water-vapor inclusions are frequently large and can be observed with the naked eye. These samples, commercially referred to as “enhydro,” are extremely rare and valuable collectibles. Numerous quartz and amethyst specimens with large aqueous inclusions and moving bubbles originate in Brandberg, Namibia, but are also found in other deposits. Water is not always the liquid phase in this type of inclusion. In Sri Lankan sapphires, two-phase liquid-vapor CO2 inclusions are common, occasionally with some additional solid phases within equivalent cavities. Gently heating such inclusions to 31.1oC is sufficient to merge them into a liquid phase.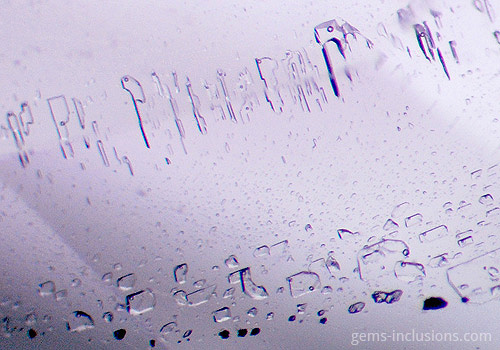
Two-phase liquid-liquid inclusions
In this case, the inclusion cavity is filled with two immiscible liquids at room temperature. In the case of liquid-vapor inclusions, heating the sample causes homogenization of the two liquids into a single fluid, corresponding to the primary fluid present during crystallization. However, cooling two liquids to room temperature separates them into two distinct phases under standard observation conditions. Water and CO2 are the most frequently encountered immiscible liquid phase combinations, as are water and liquid hydrocarbons (petroleum). True primary liquid-liquid inclusions are uncommon due to the presence of a gaseous phase bubble within the same cavity. However, liquid-liquid inclusions can also form as a result of the “necking down” of larger liquid-liquid-vapor inclusions, which occurs when a primary large cavity is divided into two or more distinct cavities and the vapor phase bubble is isolated to at least one of them, leaving only two immiscible liquids in the remaining cavities. Necking down is a common feature of fluid inclusions of all types; it occurs as a result of the modification of the borders of a primary cavity, with the host crystal dissolving in some areas and dissolved material reprecipitating in others. It frequently forms more energy-efficient negative-crystal-shaped cavities with borders that correspond to the host crystal’s faces. This process, which results in the formation of negative crystal cavities, can also result in the complete separation of one part of a large inclusion from the other via a barrier of reprecipitated material.
Two-phase liquid-solid inclusions
Due to two distinct phenomena, a solid phase is frequently present within a fluid inclusion cavity: • Occasionally, a solid particle is entrapped during crystal growth (captured crystal). • When the trapped fluid is cooled to space temperature, chemical compounds dissolved within it precipitate. Only in the second case is the solid phase frequently referred to as a “daughter mineral,” as it forms as a result of precipitation of the trapped fluid. In contrast to daughter phases formed from homogeneous fluid after the system (inclusion cavity) was closed, any phase (solid, liquid, or vapor) incorporated in an inclusion during crystal growth and fluid entrapment is referred to as a captured phase. Tiny crystals of various protogenetic and/or syngenetic minerals are frequently suspended in the fluid during mineralization and thus become trapped inside inclusion cavities along with the fluid. Additionally, particles of these minerals are frequently deposited on the growing crystal’s surface and act as a barrier to crystal growth, resulting in the formation of fluid inclusions adjacent to solid inclusions. As a result, solid phases are frequently found within the fluid inclusion cavity as well, but they have captured minerals rather than daughter phases. Daughter minerals are formed by highly soluble compounds; they also occur as crystals in all primary inclusions of an equivalent assemblage (unless necking down took place). They are relatively small in comparison to the total volume of inclusions, and thus the relationship between liquid and solid phase volumes is consistent for all inclusions of an equivalent group. The inclusion’s heating will result in the dissolution of daughter minerals, while cooling will result in their precipitation, preserving the solid phase’s initial volume. By contrast, captured minerals are found in only a few fluid inclusions of the same group, they have a tendency to have partially dissolved irregular shapes, their volume is highly variable, they are extremely large in comparison to the volume of the fluid inclusion, and in many cases, they will completely overpass the fluid inclusion’s borders. Heating fluid inclusions to the temperature at which all other phases are homogenized will not dissolve captured crystals. True primary two-phase liquid-solid inclusions are uncommon because they are typically accompanied by a vapor bubble, resulting in a typical three-phase liquid-solid-vapor inclusion. Such inclusions are characteristic of Colombian emeralds, for example. Nonetheless, some two-phase solid-liquid inclusions can form as a result of the “necking down” phenomenon, as described previously in the section on two-phase liquid-liquid inclusions.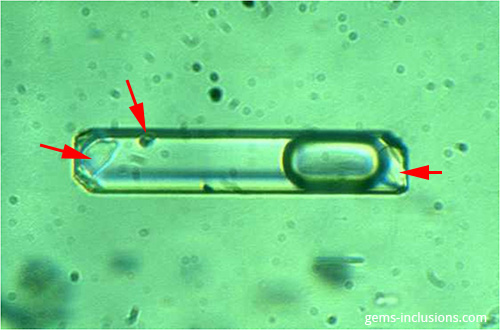
Field of view 0.15 mm.
Two-phase solid-gaseous inclusions
When observed at room temperature, we see a solidified glass with gas bubbles; it is no longer a fluid inclusion, but it was at the time of entrapment. These inclusions are characteristic of synthetic materials crystallized using the flux method (emerald, ruby, sapphire, alexandrite, spinel). A molten substance (flux) is used as a solvent for the crystallization of the mineral’s components and as a medium for crystallization in this process. While portions of flux are liquid when trapped by the growing crystal, cooling to room temperature causes them to solidify and contract, forming a small vacuum bubble within each cavity. As a result, we observe inclusions that are similar to natural gems’ two-phase liquid-vapor inclusions. The same process occurs in some gemstones that have been treated. For example, in heat-treated corundum (rubies and sapphires), when heating is carried out in the presence of borax or other fluxing agents with the intention of “healing” natural crystal fractures. These substances melt, enter open fractures, and act as a solvent for the host crystal, resulting in its recrystallization and fissure “healing.” Naturally, some flux material is trapped within the fissures and, when cooled, forms solid inclusions with bubbles, in a way comparable to that described previously for flux synthetics. Indeed, the appearance of inclusion veils in heat-treated corundum is frequently very similar to that of flux-grown crystals. When emeralds are treated with artificial resins to fill their fractures and cavities, trapped gas bubbles can occasionally be seen, as in the image below, where solidified resin fills a large cavity formed by the dissolution of a mineral inclusion (pyrite or calcite), and a large bubble is frequently visible within the filling material. Finally, two-phase solid-gaseous inclusions can be formed through heat treatment and subsequent melting of solid inclusions within the gem. Rubies and sapphires can withstand extremely high temperatures during heat treatment. Corundum has a melting point of 2044oC and can be heated to 1800oC. When the melting point of minerals contained in heat-treated stones is less than the heating temperature, they will melt and, in some cases, decrepitate, forming circular halos of solidified glass. In other cases, melted and solidified material will remain contained within the initial solid inclusion’s equivalent cavity. In any case, two-phase solid-vapor inclusions of solidified glass with bubbles are frequently formed as a result.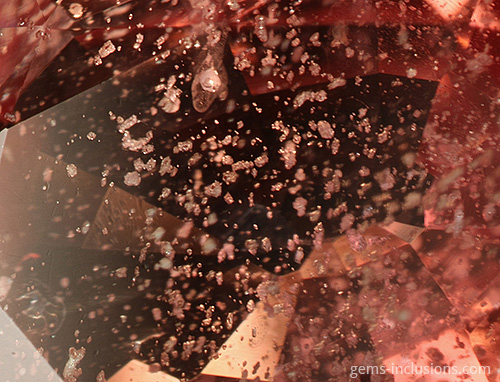




Three-Phase Inclusions
Three-phase inclusions are typically formed when a homogeneous fluid trapped in a cavity cools to room temperature and separates into three distinct phases. The most common types of inclusions are liquid-solid-vapor and liquid-liquid-vapor inclusions, which contain two immiscible liquids. Naturally, captured phases can also exist in three-phase inclusions, as discussed in the section titled “Two-phase liquid-solid inclusions.” Gemologists are very familiar with three-phase liquid-solid-vapor inclusions as they are characteristic of Colombian emeralds. In this case, the primary fluid is saturated with sodium chloride, and when the emerald crystal is cooled to room temperature from the temperature at which it formed (290-360oC, according to Cheilletz et al., 1994), a cubic crystal of sodium chloride crystallizes within the inclusion cavity, a so-called “daughter mineral” precipitated from the solution trapped as fluid inclusion. Aqueous fluid also separates into two phases as a result of cooling – liquid and vapor phases, as described in the section on two-phase liquid-vapor inclusions. When such inclusions are heated, complete homogenization of all three phases is frequently observed as a result of the salt crystal dissolving and the vapor bubble fading away. There is another type of three-phase inclusion that is quite common: those that contain two immiscible liquid phases and a vapor bubble. Water and CO2 are the two immiscible liquids most frequently found in inclusions, but water and liquid carbon hydrates (petroleum) are also quite common. As with other types of fluid inclusions, the fluid is homogeneous at the time of entrapment but separates into three distinct phases upon cooling to room temperature. When heated to the homogenization temperature, a single-phase fluid similar to that trapped during crystal growth will be produced. UV light is frequently used to detect liquid petroleum contained in fluid inclusions. In comparison, liquid CO2, which is present in a large number of inclusions, is occasionally more difficult to examine. Because liquid CO2 has a lower density than water, it is always concentrated near the gas bubble, forming a new phase between water and vapor. If the amount of liquid CO2 is significant, a double rim can easily be seen around the gas bubble, sometimes in the shape of a crescent moon. In comparison, a trace of liquid CO2 is frequently difficult to observe; at times, it merely makes the rim between water and vapor appear thicker and more defined. Cooling such inclusions increases the amount of visible liquid CO2, whereas slight heating above 31.1Co always results in homogenization of the liquid and vapor CO2, converting the inclusion to a two-phase liquid-vapor type. Video footage of microthermometric study of fluid inclusion in emerald from the Urals, Russia.Multi-phase inclusions
Multi-phase inclusions are any combination of inclusions that exhibit at least three distinct phases at room temperature. Such inclusions are extremely uncommon in nature. Several examples of this type of inclusion include the following: Inclusions containing multiple daughter mineral phases, as well as water and vapor bubbles. Two or more distinct dissolved compounds are precipitated from the trapped fluid within the inclusion cavity in this case. Two immiscible liquids are added to the mix, along with a solid phase and a gas bubble. For example, in many inclusions containing water and petroleum, solid carbon hydrates (bitumen particles) are frequently observed floating in the water alongside the gas bubble. Multi-phase inclusion in quartz from Pakistan, with yellow petroleum, a small amount of water in the lower right part, solid bitumen particles, and vapor bubble. Field of view 7 mm.
Multi-phase inclusion in quartz from Pakistan, with yellow petroleum, a small amount of water in the lower right part, solid bitumen particles, and vapor bubble. Field of view 7 mm.



